What are the subatomic particles that move around the nucleus of an atom?
Do x-rays have the same frequency as microwaves?
About this website
Do atoms of the same group have the same number of shells?
This similarity occurs because the members of a group have the same number and distribution of electrons in their valence shells. However, there are also other patterns in chemical properties on the periodic table.
Which elements have the same number of electron shells?
Elements in each period have the same number of electron shells as the number of that period. Hydrogen and helium are in period 1, and both have one electron shell. Carbon, nitrogen and oxygen are all in period 2, and all have two electron shells. And so on.
What do all atoms of the same element share in common?
The fundamental characteristic that all atoms of the same element share is the number of protons. All atoms of hydrogen have one and only one proton in the nucleus; all atoms of iron have 26 protons in the nucleus.
What do electron shells represent?
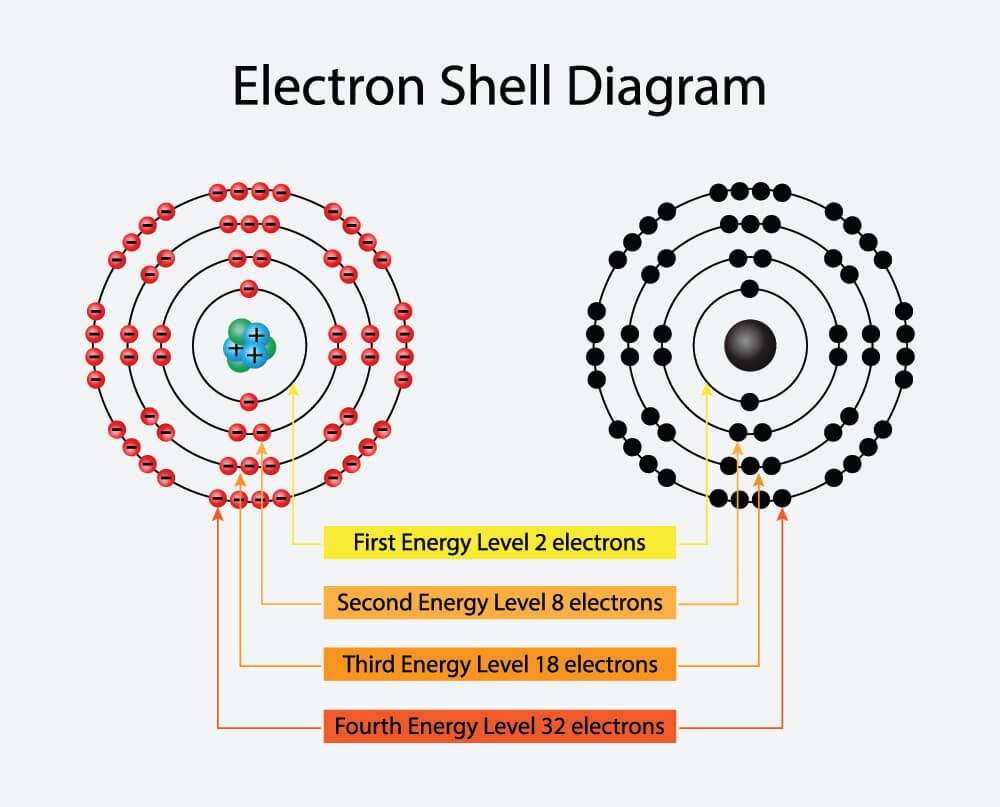
An electron shell may be thought of as an orbit followed by electrons around an atom nucleus. Because each shell can contain only a fixed number of electrons, each shell is associated with a particular range of electron energy, and thus each shell must fill completely before electrons can be added to an outer shell.
What do elements in the same group have in common?
The elements in each group have the same number of valence electrons. As a result, elements in the same group often display similar properties and reactivity.
Do electrons in the same shell of an atom have the same energy?
Electron shells consist of one or more subshells, and subshells consist of one or more atomic orbitals. Electrons in the same subshell have the same energy, while electrons in different shells or subshells have different energies.
What do all the atoms have in common?
The common feature is that the atoms of all elements consist of electrons, protons, and neutrons.
What do all atoms within the same element have that is the same?
All atoms of the same element have the same number of protons, but some may have different numbers of neutrons. For example, all carbon atoms have six protons, and most have six neutrons as well.
How do electrons in the same atom differ?
The electrons do NOT orbit around the nucleus like planets around the sun. All of the atoms of a particular element have the same number of protons, but could differ in the number of neutrons or the number of electrons. If the number of electrons changes, the atom becomes a charged particle and is called an ion.
How are electrons arranged in shells?
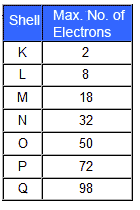
Each shell can contain only a fixed number of electrons: the first shell can hold up to two electrons, the second shell can hold up to eight (2 + 6) electrons, the third shell can hold up to 18 (2 + 6 + 10) and so on. The general formula is that the nth shell can in principle hold up to 2(n2) electrons.
Why are there only 2 electrons in the first shell?
This first shell has only one subshell (labeled 1s) and can hold a maximum of 2 electrons. This is why there are two elements in the first row of the periodic table (H & He). Because the first shell can only hold a maximum of 2 electrons, the third electron must go into the second shell.
How many electrons are in each shell?
The first shell (closest to the nucleus) can hold two electrons. The second shell can hold 8 electrons. The third shell can hold 32 electrons. Within the shells, electrons are further grouped into subshells of four different types, identified as s, p, d, and f in order of increasing energy.
Which group of elements have the same number of electron orbitals?
The elements in each group have the same number of electrons in the outer orbital. Those outer electrons are also called valence electrons. They are the electrons involved in chemical bonds with other elements. Every element in the first column i.e. group one has one electron in its outer shell.
Which two elements have the same number of electrons in the 3d shell?
Chromium and manganese thus have the same number of 3d electrons: five.
How do you determine the number of electron shells?
1:242:184.1l Counting the electron shells in a neutral atom - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo for its outermost electrons and equals six that means it has N equals five four three two and oneMoreSo for its outermost electrons and equals six that means it has N equals five four three two and one or a total number of six electron shells.
Does lithium have 2 electron shells?
A lithium atom, for example, has three electrons. It has two in the first shell and one in the second shell....Electron shells.Energy shellMaximum number of electronsFirst2Second8Third8
What are some exceptions to the d block?
Exceptions. Elements of d-block and f-block have some exceptions. These elements do not show the same number of outermost electrons as that of their group number. For example, 3rd group elements should have 3 valence electrons, but they have only 2 valence electrons. Similarly, 4th group elements should have 4 valence electrons, ...
What are the vertical columns in the periodic table called?
Modern periodic table consist of groups and periods. The vertical columns are called groups. The elements which are in the same groups have the same number of valence electrons (i.e same number of electrons in the outermost shell). Also they possess almost similar properties.
How many valence electrons does each element have?
All the elements of 1st group (H, Li, Na, K, Rb, Cs, Fr) have one valence electron.
How many electrons are in the 13th group?
All the elements of 13th group have 3 electrons in its outermost orbit.
How many electrons are in the outer shell of a 14th group?
14th group elements have 4 electrons in outer shell, not 14.
Which group of elements has the same number of valence electrons?
Elements of group 17 are highly electronegative and have higher ionisation enthalpy. They react with water and form halides. Thus all the elements of group 17 have the same number of valence electrons (i.e 7) and they show common properties.
Do d-block elements have orbitals?
This is because the d-block elements have outermost electrons in s-orbitals as well as d-orbitals. (If you do not know about orbitals, visit here) Also these d-block elements have incomplete d-orbitals. Thus electrons of both s-orbitals as well as d-orbitals participate in chemical reaction.
How much does an atom of oxygen weigh?
An atom of oxygen weighs 8 grams.
What is the building block of matter?
It proves that atoms are the building blocks of matter.
Can atoms be separated?
They cannot be separated once they have bonded with other atoms.
Is it harder to split atoms?
It would be more difficult to split atoms.
What are the subatomic particles that move around the nucleus of an atom?
Electrons are said to be the subatomic particles that move around the nucleus of an atom. These electrons are negatively charged particles that are seen to be quite smaller than the nucleus of an atom.
Do x-rays have the same frequency as microwaves?
What can you conclude about x-rays compared to microwaves? X-rays have the same frequency as microwaves. X-rays have a lower frequency than microwaves. X-rays have a longer wavelength than microwaves. X-rays have a shorter wavelength than microwaves.