What are osteoprogenitor cells?
Osteoprogenitor cells, also known as osteogenic cells, are stem cells located in the bone that play a prodigal role in bone repair and growth. These cells are the precursors to the more specialized bone cells (osteocytes and osteoblasts) and reside in the bone marrow.
How do osteoblasts differentiate into osteocytes?
These components are activated by chemical signals from local cells and serum hormones such as vitamin D metabolites and parathyroid hormone (PTH). The lifespan for osteoblasts is typically around 6 months and 10–15% of them become surrounded by bone and differentiate into osteocytes, while others transform into bone lining cells.
What is the function of perivascular osteoprogenitors?
Perivascular osteoprogenitors are associated with transcortical channels of long bones. [Stem Cells. 2020] Perivascular osteoprogenitors are associated with transcortical channels of long bones.
Which hormones are most important for early osteogenesis?
Among the hormones most influencing early osteogenesis are parathyroid hormone (PTH) which stimulates growth of osteoprogenitor populations [ 181 ], and glucocorticoids that have been shown to stimulate the growth and differentiation of CFUs and osteoprogenitors from human and rat, but not the mouse [ 182 ].
What are osteoprogenitor cells?
Where are osteoprogenitor cells located?
Which type of osteoprogenitors are associated with transcortical channels of long bones?
What is MSC in bone marrow?
See 1 more
About this website

What do osteoprogenitor cells do?
Osteoprogenitor cells are the 'stem' cells of bone, and are the source of new osteoblasts. Osteoblasts, lining the surface of bone, secrete collagen and the organic matrix of bone (osteoid), which becomes calcified soon after it has been deposited.
What do osteogenic cells give rise to?
Osteogenic cells differentiate and develop into osteoblasts which, in turn, are responsible for forming new bones. Osteoblasts synthesize and secrete a collagen matrix and calcium salts.
Which cell type gives rise to osteoblasts?
Osteoprogenitor cells residing in the bone marrow give rise to osteoblasts that progress through a series of maturational stages resulting in the mature osteocyte.
Where are osteoprogenitor cells?
Osteoprogenitor cells isolated from bone and periosteum have been cultured on porous scaffolds to form bone-like tissue. The most widely used cells for tissue engineering of bone are adult mesenchymal stem cells (MSCs), which are multipotent and proliferative.
What is the function of osteogenic cells quizlet?
Terms in this set (12) What is the function of the osteogenic cells and where? Develops into an osteoblast.
Where do osteogenic cells come from?
Osteoprogenitor cells (AKA osteogenic cells) are the stem cells found in bone tissue. Specialized bone cells (i.e., the osteoblasts and osteocytes) originate from osteoprogenitor cells in the bone marrow.
What are osteogenic cells?
Osteoprogenitor cells, also known as osteogenic cells, are stem cells located in the bone that play a prodigal role in bone repair and growth. These cells are the precursors to the more specialized bone cells (osteocytes and osteoblasts) and reside in the bone marrow.
What is an osteogenic?
Medical Definition of osteogenic 1 : of, relating to, or functioning in osteogenesis especially : producing bone the osteogenic layer of the periosteum. 2 : originating in bone.
Osteoprogenitor Cell - an overview | ScienceDirect Topics
Johnathan Ng, ... Gordana Vunjak-Novakovic, in Biology and Engineering of Stem Cell Niches, 2017. 2.2 Sources of Autologous Cells for Bone Formation. Osteoprogenitor cells isolated from bone and periosteum have been cultured on porous scaffolds to form bone-like tissue. 17–19 The most widely used cells for tissue engineering of bone are adult mesenchymal stem cells (MSCs), which are ...
Histology, Osteoprogenitor Cells - PubMed
Osteoprogenitor cells, also known as osteogenic cells, are stem cells located in the bone that play a prodigal role in bone repair and growth. These cells are the precursors to the more specialized bone cells (osteocytes and osteoblasts) and reside in the bone marrow. Developed from infant mesenchym …
Osteoprogenitor cells | definition of ... - Medical Dictionary
osteoprogenitor cells: the stem cells of the stromal system, the only cells capable of independent osteogenesis. Called also osteogenic precursor cells.
Osteoprogenitor cell | definition of ... - Medical Dictionary
osteoprogenitor cell: one of the cells in the inner layer of the periosteum that develop into osteoblasts. Synonym(s): osteoprogenitor cell , preosteoblast
The Expanding Life and Functions of Osteogenic Cells: From Simple Bone ...
During the last three decades, important progress in bone cell biology and in human and mouse genetics led to major advances in our understanding of the life and functions of cells of the osteoblast lineage. Previously unrecognized sources of osteogenic cells have been identified. Novel cellular and …
What are osteoprogenitor cells?
Osteoprogenitor cells can arise from stem cells in a variety of tissues. There are as yet no unique identifying markers for the mesenchymal stem cell (MSC) that gives rise to bone, fat, cartilage, and muscle. Bone marrow stroma contains highly proliferative cells that will form single colonies or colony-forming units-fibroblasts (CFU-Fs) and are thought to contain mesenchymal stem cells that can be distinguished from early hematopoietic precursors. 62–65 Stromal cells grown in vitro are heterogeneous with respect to the capacity for differentiation, and only a low percentage of all CFU-Fs have stem cell properties. 62,63,66,67 Further, only a small fraction of CFU-Fs are bone-forming cells. 68,69 Although a specific osteoprogenitor marker has not been identified, antibodies have been developed (e.g. STRO-1, SP-10, SH2, HOP-26) that recognize some subsets of precursor cells. 70–77
What are the cells that give rise to bone?
Osteoprogenitor cells. Osteoprogenitor cells can arise from stem cells in a variety of tissues. There are as yet no unique identifying markers for the mesenchymal stem cell (MSC) that gives rise to bone, fat, cartilage, and muscle.
What differentiates osteoblasts from mesenchymal stem cells?
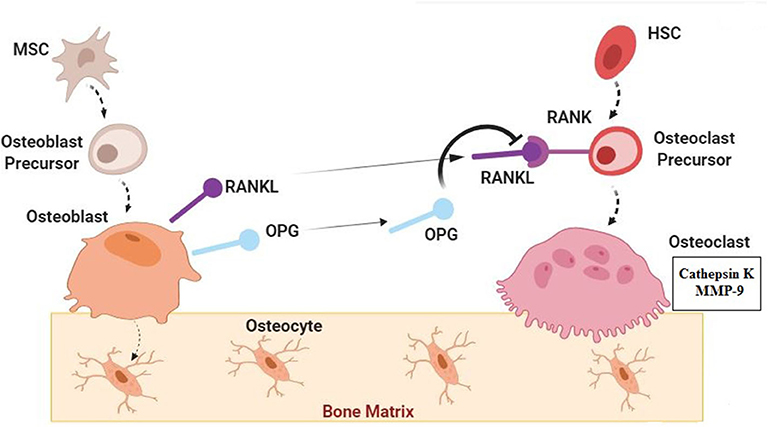
Osteoprogenitor cells differentiate from mesenchymal stem cells in bone marrow. Bone marrow can, therefore, be seen as a reservoir of osteoprogenitor cells, which differentiate further into pre-osteoblasts, which then develop into mature osteoblasts on reaching the substrate onto which bone will be deposited. There are numerous osteoprogenitor cells in the marrow of young, growing individuals, but either their number or their potential to differentiate further to mature osteoblasts declines considerably with age. The origin of osteoblasts recruited for bone synthesis later in life remains unclear, but there is evidence that these stem cells are also present in the membranes surrounding bones—the periosteum and endosteum (Figure 2-1). Osteoblasts are responsible for the formation of osteoid, the unmineralized component of bone tissue. Osteoblasts contain the normal basic ingredients of a cell-a single nucleus, endoplastic reticulum, well-developed Golgi bodies, and numerous ribosomes and mitochondria—reflecting the requirement for abundant protein synthesis in osteoid formation (Figure 2-2). These proteins include pro-α collagen (a principle component of collagen), osteocalcin, and bone morphogenic proteins. Differentiation of osteoblasts is stimulated by transforming growth factor and bone morphogenic proteins. These components are activated by chemical signals from local cells and serum hormones such as vitamin D metabolites and parathyroid hormone (PTH). The lifespan for osteoblasts is typically around 6 months and 10–15% of them become surrounded by bone and differentiate into osteocytes, while others transform into bone lining cells.
How do osteocytes sense bone?
There is growing evidence that the mechanical loading of bone is sensed by osteocytes via extracellular fluid being forced through the network of canaliculi. This fluid, which carries charged ions, generates streaming potentials that stimulate the osteocytes and they in turn send electrochemical or hormonal signals to other cells involved in bone maintenance and remodeling. Osteocytes also appear to regulate the flow of calcium and phosphorus into and out of bone matrix under stimuli from thyroid hormone (calcitonin) and PTH. Osteocytes may live for several years before being replaced as part of the normal mechanism of bone remodeling, which may in some instances be stimulated by osteocyte apoptosis or programmed cell death.
What are osteoclasts responsible for?
Osteoclasts are responsible for the breakdown and resorption of bone tissue. They differ markedly from osteoblasts in that they are very large, irregularly shaped cells with more than one nucleus ( Figure 2-4 ). There are no cytoplasmic extensions from the cell, which contains many more lysosomes, reflecting its potential for destruction of bone tissue. Mitochondria and the endoplasmic reticulum are minor components, indicating that protein synthesis by osteoclasts is minimal. Osteoclasts arise from the fusion of several blood-borne mononuclear macrophage precursors, which in turn are derived from hematopoetic stem cells in bone marrow under stimulation from vitamin D 3 (see subsequent text). They are very active, motile cells and move around the resorbing surfaces of bone. Furthermore, osteoclasts frequently form clusters of cells during resorption and in histological sections of bone several may be seen occupying eroded depressions in the surface known as Howship's lacunae ( Figure 2-5 ). Osteoclasts adhere to bone surfaces via intracellular contractile proteins attached to integrins, which are specialized cell surface receptors ( Vaes 1988 ). This leads the mature osteoclast to form a ruffled border that allows a high surface area in contact with the bone surface. The depressions or lacunae formed by one cell may be removed by the activity of another osteoclast. This may complicate interpretation of bone osteoclast activity, but Howship's lacunae are almost always present if bone resorption has occurred relatively recently before death. Since these cells are absent in archeological bone specimens, one microscopic indicator of active bone resorption is the presence of Howship's lacunae.
What are the basic components of an osteoblast?
Osteoblasts contain the normal basic ingredients of a cell-a single nucleus, endoplastic reticulum, well-developed Golgi bodies, and numerous ribosomes and mitochondria— reflecting the requirement for abundant protein synthesis in osteoid formation ( Figure 2-2 ).
How do osteoclasts adhere to bone?
Osteoclasts adhere to bone surfaces via intracellular contractile proteins attached to integrins , which are specialized cell surface receptors ( Vaes 1988 ). This leads the mature osteoclast to form a ruffled border that allows a high surface area in contact with the bone surface.
What are the stages of differentiation of osteoprogenitor cells?
The differentiation of osteoprogenitor cells and MSCs is characterized by several well-defined stages: (1) proliferation, characterized by cell multiplication and the synthesis and deposition of an osteogenic matrix containing predominantly collagen type I fibers; (2) matrix maturation, associated with the increased expression of several osteogenic genes, such as alkaline phosphatase (AP); and (3) matrix calcification, occurring with the deposition of calcium phosphate crystals and the expression of additional late osteogenic markers, such as bone sialoprotein and osteocalcin.58
What genes are involved in osteogenic differentiation?
59 Furthermore, quantitative real-time polymerase chain reaction (PCR) confirms enhanced expression of bone morphogenic protein-2 (BMP2), bone sialoprotein, osteopontin, and CBFA-1 on osteogenic induction of human MSCs. 81 Consistent with this, osteogenic induction of PLA cells results in the expression of CBFA-1 and collagen type I mRNA, in addition to osteonectin, osteopontin, and AP at both the gene and protein level. Moreover, real-time quantitation of CBFA-1 and AP gene expression during differentiation reveals a significant time-dependent increase in their expression. 37 Although increased expression of genes like CBFA-1 and AP are indicative of osteogenic capacity, it is not absolute proof. For this, one requires osteogenic-specific genes, such as bone sialoprotein and osteocalcin. 58 Increased expression of bone sialoprotein is observed in mineralizing osteoblasts, 82 embryonic stem (ES) cells, 83 and osteo-induced MSCs, 81 consistent with its role in mediating attachment of terminally differentiated osteoblasts to the extracellular matrix. 84 Although bone sialoprotein expression is absent in noninduced PLA cells, it is expressed at significant levels in PLA cells treated with osteogenic factors in combination with BMP-2. 85 Like bone sialoprotein, osteocalcin is a late marker of differentiation in osteoblasts; however, it is expressed specifically during the early stages of MSC osteogenesis. 59 Like MSCs, PLA cells also express osteocalcin during both the early and later stages of osteogenesis. 37 However, distinctions in osteocalcin expression kinetics appear to differ between PLA and MSCs. First, researchers have reported constitutive expression of osteocalcin in MSCs. 86 No such basal level can be observed in PLA cells. Second, osteocalcin expression appears to be more sensitive to induction conditions in PLA cells versus MSCs. Although 1,25-dihydroxyvitamin D 3 treatment induces osteocalcin expression in both stem cell populations, dexamethasone-induced MSCs express lower levels 37,73,87,88 and complete inhibition of this gene is seen in similarly induced PLA cells.
How does integrin affect osteoblasts?
Attachment to a material surface via integrin binding influences osteoprogenitor cell migration, adhesion, and spreading. The chemical and physical properties of the surface direct adsorption of proteins sensed by these cells. Integrin mediated signaling influences cell behavior, osteoblast differentiation and osteogenesis. ECM components such as collagen and fibronectin are important in stimulating osteoblasts and directing bone growth. Recent strategies have enhanced the therapeutic function of these tissue components by creating peptide mimetics based on amino acid motifs found in ECM proteins like collagen, fibronectin, and bone sialoprotein.113–115 By adjusting the presentation and surface density of these peptides, osteoblast attachment, adhesion, migration, and differentiation can be tailored. These specific peptide sequences can also provide more direct stimulatory signaling while reducing infiltration of non-osteogenic cells.
What is the fate of an osteoblast?
The differentiating osteoblast has one of three fates: It can become embedded in its own osteoid and continue differentiation into an osteocyte; it can quiesce into a lining cell; or more likely, it can undergo apoptosis (for review, see Manolagas [ 6 ]). Karsdal and coworkers proposed that matrix metalloproteinase activation of latent transforming growth factor β (TGF-β) blocks osteoblast apoptosis, thereby delaying differentiation into osteocytes [ 7 ]. Identification of mechanisms responsible for osteoblast apoptosis has implications for development of strategies to reduce or inhibit osteoblast apoptosis that could potentially increase bone mass. However, inhibition of osteocyte apoptosis may have beneficial or nonbeneficial effects on bone depending on condition, as addressed later in this review. (See Figure 8-2 .)
Where do osteoblasts live?
Osteoprogenitor cells residing in the bone marrow give rise to osteoblasts that progress through a series of maturational stages resulting in the mature osteocyte. Biomarkers and functional assays have been used to discriminate between these various stages. Whereas numerous markers for osteoblasts are available (cbfa1, osterix, alkaline phosphatase, collagen type I, osteocalcin, etc., see also Chapter 9 ), few markers have been available for osteocytes until recently. It would be expected that osteocytes would share some markers with their progenitors – osteoblasts, but would also express unique markers based on their morphology and potential function. Kalajzic and coworkers have used promoters for osteocalcin and collagen type I linked to green fluorescent protein (GFP) to examine transgene expression during osteoblast differentiation [5]. Osteocalcin-GFP was expressed in a few osteoblastic cells lining the endosteal bone surface and in scattered osteocytes whereas GFP driven by the collagen type I promoter was strongly expressed in osteoblasts and osteocytes. These investigators also generated an osteocyte-selective promoter, the 8kb dentin matrix protein 1 (DMP1) driving GFP that showed exclusive expression in osteocytes [6].
What is the process of ossification of the shaft of the long bone called?
The process of ossification of the shaft of the long bone is sometimes called primary ossification; the original focus of ossification is referred to as the primary ossification center. The area of first vascular ingrowth into the bone becomes the nutrient artery of the bone shaft (diaphysis).
Why is collagen not a layering arrangement?
The layering arrangement of collagen in lamellar bone is not because there are actually successive layers of collagen that can be separated from one another; in fact all of the bone collagen is one contiguous piece of material. It is created because the tightly packed, parallel collagen fibers interchange their orientation in successive lamellae such that when a section of bone is viewed in polarized light, when there is maximal brightness visible in one layer of collagen, there is maximal light extinction in the adjacent lamella ( Figure 5, top). The actual orientation of the collagen fibers in successive lamellae can also be visualized in bright field microscopy if a reticulin stain is substituted for hematoxylin and eosin ( Figure 5, bottom).
What are osteoprogenitor cells?
Osteoprogenitor cells are often referred to as preosteoblasts. They can be present within the endosteum, the cellular layer of the periosteum, and the lining of the osteogenic cells. In matured bones that no longer display active bone remodeling or formation, osteoprogenitor cells exist as flattened spindle-shaped structures. They attach to the bone surface and are referred to as “inactive osteoblasts” during this period. In maturing bones, however, these cells appear in their largest form. During fetal development or high turnover periods in adult osteogenesis, numerous osteoprogenitor cells function to give rise to osteoblasts. At this stage, these structures display plump oval nuclei and emboldened abundant spindle-shaped cytoplasm, converting later to characteristic cuboidal active osteoblasts. [2][3]
Where are osteoprogenitor cells located?
Osteoprogenitor cells, also known as osteogenic cells, are stem cells located in the bone that play a prodigal role in bone repair and growth. These cells are the precursors to the more specialized bone cells (osteocytes and osteoblasts) and reside in the bone marrow. Developed from infant mesenchymal cells, osteoprogenitor cells turn into spindle cells at the surface of matured bones. In developing bones, they appear more frequently and activate multifunctional stages to remodel the bones. The body loses the ability to synthesize or utilize more osteoprogenitor cells with age. Dysfunction of osteoprogenitor cells may delay ossification and lead to a spectrum of diseases such as dwarfism and Kashin-Beck disease. [1]
Which type of osteoprogenitors are associated with transcortical channels of long bones?
Perivascular osteoprogenitors are associated with transcortical channels of long bones.
What is MSC in bone marrow?
Mesenchymal stem cells (MSCs) collected from adipose and bone marrow tissue hold therapeutic value for various bone disease treatments. Current studies demonstrate the benefit of bone grafts based on combinations of MSC, biomimetic scaffolds, and growth factor delivery, which showed an increased osteogenic regeneration rate with minimal side effects. The specific mechanisms of cellular signaling in bone remodeling are important in understanding the incorporation of newer effective treatment methods for numerous bone diseases.
