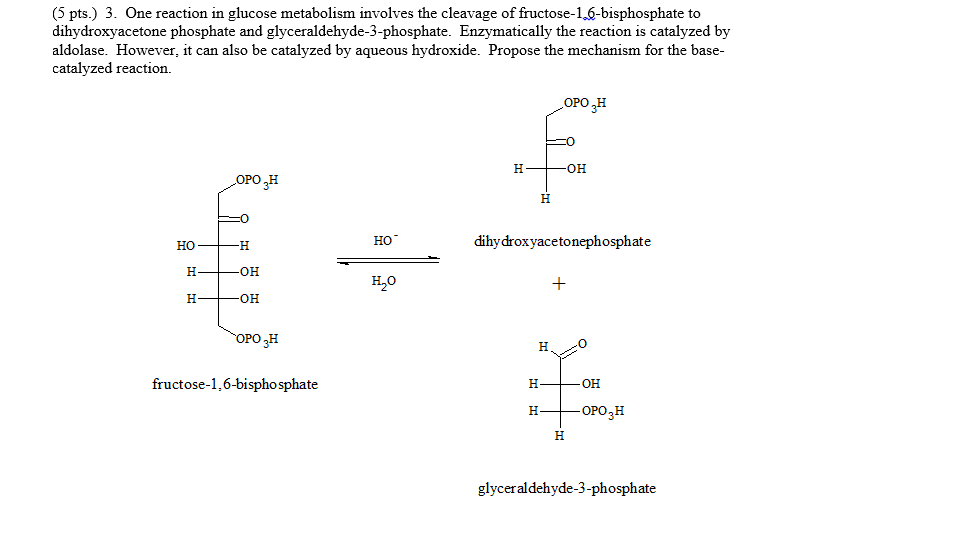
How to draw Fischer projections properly?
How to draw Fischer projections By considering a molecule with a single chiral center, e.g. a carbon atom, for drawing the Fischer projections, the tetrahedral structure is rotated so that two groups point downward, whereas two groups point upwards.
How to read Fischer projection?
numbered carbon in the Fischer projection. If the hydroxyl group (or amino group for amino acids) is pointing to the right in the Fischer Projection, the sugar (or amino acid) is designated as D. If the hydroxyl group (or amino group for amino acids) is pointing to the left in the Fischer projection, the sugar (or amino acid) is designated as L.
What type of projection is the mecator projection?
The Mercator projection is a cylindrical projection that was developed for navigation purposes. The Mercator projection was used for its portrayal of direction and shape, so it was helpful to the sailors of that time.
What is the law of projection?
The Law of Projection Everything you see outside of yourself is a projection of how you feel about you projected onto someone else or something else. The other person or object is holding up a mirror for you to see yourself and your inner feelings about yourself, more clearly. You project your fears onto the world.
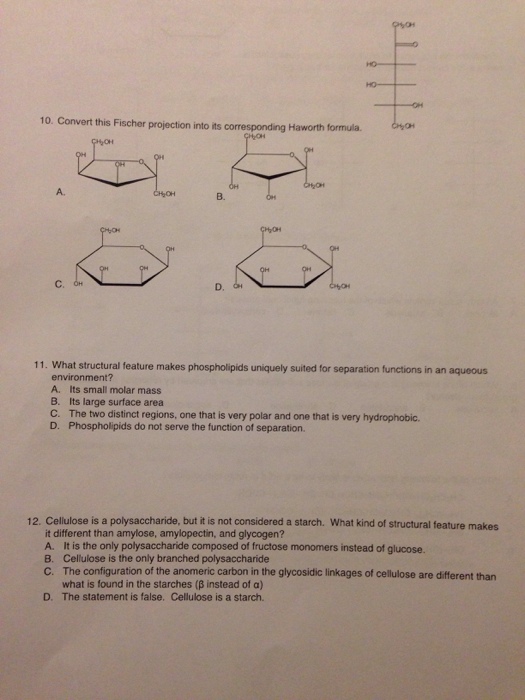
What is Fischer projection in organic chemistry?
In chemistry, the Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and biochemistry.
What are Fischer?
Fischer Projections are abbreviated structural forms that allow one to convey valuable stereochemical information to a chemist (or biochemist) without them having to draw a more detailed 3D structural representation of the molecule.
What is meant by projection formula?
Definition of projection formula : a perspective formula projected so as to represent it in two dimensions — compare structural formula.
How do you identify Fischer projections?
1:0712:40Fischer Projections How to Draw and Interpret for Single and ... - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe'll start with something as simple as a molecule with one chiral Center recall that in order for aMoreWe'll start with something as simple as a molecule with one chiral Center recall that in order for a molecule to be chiral it has to have an asymmetric Center which is typically going to be a carbon
What are Fischer and Haworth projection?
Fischer and Haworth projections are two types of illustration which are used to represent the 3D arrangement of atoms in carbohydrates. They are also used to compare different carbohydrates.
How are Fischer projections arranged?
In a Fischer projection, the longest chain is drawn vertically. The horizontal lines indicate the bonds with hydrogen, hydroxyl, and amino groups. The four bonds to a chiral carbon make a cross, with the carbon atom at the intersection of the horizontal and vertical lines.
What is the use of projection formula?
Projection formulae is the length of any side of a triangle is equal to the sum of the projections of other two sides on it. Note: We observe in the above diagram BD and CD are projections of AB and AC respectively on BC. Now, substitute the value of BD and CD in equation (ii) we get, a = b cos C - (-c cos B)
What is projection rule?
Explanation: The geometrical interpretation of the proof of projection formulae is the length of any side of a triangle is equal to the algebraic sum of the projections of other sides upon it. In any triangle ABC, (i) a = b cos C + c cos B. (ii) b = c cos A + a cos C.
What is length of projection?
What is the length of vector projection? You can find the length of the projection of a vector a onto the vector b using the formula a·b / |b| , where a·b is the dot product and |b| is the length of the vector b (the one onto which we project).
What is the difference between chiral and achiral?
Chirality and Symmetry. All objects may be classified with respect to a property we call chirality (from the Greek cheir meaning hand). A chiral object is not identical in all respects (i.e. superimposable) with its mirror image. An achiral object is identical with (superimposable on) its mirror image.
How do you draw bond line structures from Fischer projections?
To convert the Fischer projection to a bond line formula you just draw a zig-zag line of six carbon atoms. Then you put an aldehyde group at C-1 and OH groups on each of the other five carbon atoms. Note that the bond line formula gives no stereochemical information.
What do you mean by optical isomerism?
What is optical isomerism? Optical isomerism is a type of stereoisomerism in which the isomers have the same molecular formula and the structural formula but differ in their direction of rotation of plane polarized light.
Where does the name Fisher come from?
"FISHER" is an occupational surname from the Old English fiscare, meaning "fisherman." FISCHER is a common German spelling. FISHER is the 95th most common surname in England. The German variant, FISCHER, is the 4th most common surname in Germany.
What is the pronunciation of Fischer?
2 syllables: "FISH" + "uh"
1. What are the Ways to Manipulate Fischer Projection?
As the up and down aspects of the chemical bonds don’t change, a Fischer projection can be rotated by 180 degrees without changing its meaning. Als...
2. How is a Fischer Projection Different From a Haworth Projection of Carbohydrates?
Both Fischer projection and Haworth projections are standard methods of illustrating the stereochemistry of carbohydrates. However, a Haworth proje...
3. What is the Fischer projection?
Fischer projection is defined as the representation of three dimensional molecules in two dimensional figures on paper by projection without removi...
4. What are the main features of Fischer projection?
The Fischer projection consists of horizontal and vertical lines. The horizontal lines represent atoms that are projected towards the viewer and th...
5. What are some examples of Fischer projection representation?
Fischer projections are generally used to represent compounds in organic chemistry and biochemistry. Monosaccharides and amino acids are most commo...
6. What is the use of Fischer projection?
Fischer projections can be used to represent chiral molecules and differentiate between pairs of enantiomers in biochemistry and organic chemistry....
7. What are the limitations of Fischer projection?
There are two limitations of the Fischer projection relating to operations performed on Fischer projection but without changing the absolute config...
What is the Fischer projection?from vedantu.com
The Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and biochemistry.
What is the difference between Haworth projection and Fischer projection?from vedantu.com
However, a Haworth projection differs from a Fischer projection as the former is used to represent carbohydrates in their cyclical form.
How to turn a stereoformula into a Fischer projection?from chem.libretexts.org
To convert this stereoformula into a Fischer projection use the following procedure: Step 1: Hold the molecule so that. the chiral center is on the plane of the paper, two bonds are coming out of the plane of the paper and are on a horizontal plane, the two remaining bonds are going into the plane of the paper and are on a vertical plane.
What is the purpose of Haworth projections?from en.wikipedia.org
Haworth projections are a related chemical notation used to represent sugars in ring form. The groups on the right hand side of a Fischer projection are equivalent to those below the plane of the ring in Haworth projections. Fischer projections should not be confused with Lewis structures, which do not contain any information about three dimensional geometry. Wedge-and-dash notation is used to represent the stereochemistry of most classes of organic compounds, with Newman projections being used to depict specific conformations of rotatable bonds of organic molecules (including but not limited to carbohydrates).
When we use the Fischer projection for a monosaccharide with more than three carbons, is there no?from vedantu.com
When we use the Fischer projection for a monosaccharide with more than three carbons, there is no way to orient the molecule in space so that all horizontal bonds are slanted towards the viewer.
Is Fischer projection discouraged?from vedantu.com
Fischer projections in non-carbohydrates are discouraged; as such, drawings are ambiguous when confused with other types of illustration.
Do you need to keep in mind the Fischer projection rules?from vedantu.com
Thus, it is essential to keep in mind the above mentioned Fischer projection rules before using them practically.
What is Fischer Projection
Fischer, a Nobel Laureate in Chemistry in 1902, devised a systematic approach for representing chiral compounds in two dimensions, known as Fischer projections or Fischer projection formulae.
How to Draw Fischer Projections
When the Fischer projections are drawn on a molecule with a single chiral centre, such as a carbon atom, the tetrahedral structure is twisted such that two groups point downward and two groups point upward.
Conventions
Horizontal or vertical lines are used to represent all non-terminal bonds. Carbon atoms are sometimes not visible and are represented by the centre of crossing lines in the vertical representation of the carbon chain.
Uses of Fischer Projection
Fischer projections are often used to depict monosaccharides in biochemistry and chemical science. They can also be utilised for amino acids or other chemical compounds, while the 2006 IUPAC guidelines discourage this.
Things to Remember
All hydrogen atoms should preferably be drawn explicitly, according to IUPAC regulations; in particular, the hydrogen atoms of the end group of carbohydrates should be present. Fischer projection differs from skeleton formulas in this aspect.
CBSE CLASS XII Related Questions
Although the details of the structure of monoclinic sulphur are not well known it probably consists ...
Why are Fischer projections useful?
Fischer projections are especially useful in d rawing carbohydrates since they contain multiple chiral centers which are more time-consuming to draw.
What can be done with a Fischer Projection and what is disallowed?
When redrawing a Fischer projection shown from a different direction, you are allowed to rotate the molecule by 180 o but not by 90 o.
Do you need to number carbons before drawing a Fischer projection?
Before getting to drawing its Fischer projection, lets number the carbons in any order (no IUPAC rules needed). Remember, numbering carbons will always be helpful no matter what you need to do with an organic structure.
Can you practice converting Fischer projections to bond-line representation?
In the following practice problems, you can practice converting Fischer projections to bond-line representation and assigning the R and S absolute configuration of chirality centers on Fischer projections.
Why use Fischer projections?
They are helpful with depicting monosaccharides because they have so many stereocenters, or carbons with four unique bonds, and the different monosaccharides are all pretty similar, they are just different about the orientation of the stereocenters. The Fischer Projection allows us to quickly see the orientation of each monosaccharide.
What do horizontal and vertical lines represent in Fischer projections?
Fischer Projections use horizontal and vertical lines to represent the 3D state, the horizontal lines represent attachments pointing out of the paper towards us and vertical lines represent attachments pointing out the back of the paper away from us. The intersection represents the central carbon.
Can Fischer projection be applied to D-lactic acid?
True | False 2. A Fischer projection can be applied to D-lactic acid since it has several stereocenters.
What is the Fischer projection?from vedantu.com
The Fischer projection, devised by Emil Fischer in 1891, is a two-dimensional representation of a three-dimensional organic molecule by projection. Fischer projections were originally proposed for the depiction of carbohydrates and used by chemists, particularly in organic chemistry and biochemistry.
What can be done with a Fischer Projection and what is disallowed?from chemistrysteps.com
When redrawing a Fischer projection shown from a different direction, you are allowed to rotate the molecule by 180 o but not by 90 o.
What is the difference between Haworth projection and Fischer projection?from vedantu.com
However, a Haworth projection differs from a Fischer projection as the former is used to represent carbohydrates in their cyclical form.
How to turn a stereoformula into a Fischer projection?from chem.libretexts.org
To convert this stereoformula into a Fischer projection use the following procedure: Step 1: Hold the molecule so that. the chiral center is on the plane of the paper, two bonds are coming out of the plane of the paper and are on a horizontal plane, the two remaining bonds are going into the plane of the paper and are on a vertical plane.
When we use the Fischer projection for a monosaccharide with more than three carbons, is there no?from vedantu.com
When we use the Fischer projection for a monosaccharide with more than three carbons, there is no way to orient the molecule in space so that all horizontal bonds are slanted towards the viewer.
Is Fischer projection discouraged?from vedantu.com
Fischer projections in non-carbohydrates are discouraged; as such, drawings are ambiguous when confused with other types of illustration.
Do you need to keep in mind the Fischer projection rules?from vedantu.com
Thus, it is essential to keep in mind the above mentioned Fischer projection rules before using them practically.
Why can a Fischer projection be rotated 180 degrees?
Because the "up" and "down" aspects of the bonds don't change, a Fischer projection may be rotated by 180 degrees without changing its meaning.
What is the wedge and dash representation of stereochemistry?
The wedge and dash representations of stereochemistry can often become cumbersome, especially for large molecules which contain a number of stereocenters. An alternative way to represent stereochemistry is the Fischer Projection, which was first used by the German chemist Emil Fischer. The Fischer Projection represents every stereocenter as a cross.
What is the application of Fischer projections?
An important application of Fischer projections is the ease of conveying the stereochemistry of carbohydrates and their conversion from Fischer, Haworth, and chair structures.
How to convert a Newman projection to a bond line?
So, first, decide the direction you are going to use. It can be any – right or left unless specified in the question.
Is Haworth projection the same as Newman projection?
It might be helpful to convert the Newman projection to Haworth before getting the final structure in the bond-line. Haworth projection is different from the Newman in that it shows the bond between the front and back carbons. So, it is not looking directly through the bond, but rather at a slightly tilted angle:
Is the Cl and Br wedge or dash?
Again the wedge and dash are relative to the direction we are projecting the molecule and if the viewer was on the right side, then the Cl and Br would’ve been wedge. The aldehyde group, however, would’ve been on the right side and the methyl on the left. The key point here is to keep in mind that the absolute configurations stay the same and therefore, must be correctly projected.
Do Fischer projections like you?
Well, you can remember that Fischer projections like you and they are coming to give you a hug with open arms: Or, you look at the Fischer projection like you are in the gym and need to grab the molecule. In this case, as well, the horizontal groups have to be pointing towards you.
Is a Newman projection the same as a bond line?
Regardless of the molecule shown in bond-line, Newman, or Fischer projection, it is still the same molecule! And therefore, it must have the same absolute configuration of all the chiral centers. This means, for example, when you are converting a Fischer projection to a bond-line, you can simply draw the zig-zag with the correct number ...
