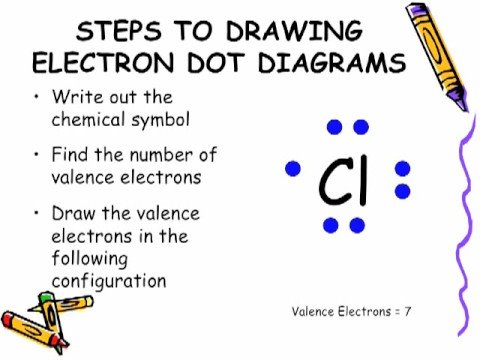
How many dots belong in the electron dot diagram?
Three dots belong in the electron dot diagram of a Boron. In drawing Lewis structures the dots presented on the atoms are the valence electrons present in the valence shell of that particular atom. Therefore, the number of valence electrons in an atom can be calculated from its electronic configuration.
What is the purpose of an electron dot diagram?
- Electron dot diagram is known Lewis dot structure.
- It is helpful in understanding structure of compound and electron in 2D about there electrons.
- I hope it may help u.
How to determine the electron dot structure?
To generate the Lewis dot structure, you have to follow the given steps:
- Find the total count of valence electrons to molecules. ...
- Find the required count of electrons needed to make the atoms complete.
- After then, define the number of bonds in the given molecule.
- Later on, choose a central atom.
- And then, draw a skeletal figure.
- After completion of the skeletal figure, place electrons around outside atoms to complete it.
What does Lewis electron dot diagram show us?
Well, the Lewis Dot Structures or the electron dot diagrams basically helps us to know how the bonds between atoms are formed. Electron dot diagram is a method of writing the chemical symbol of an element by surrounding it with dots to indicate the number of valence electrons.
.PNG)
What electrons do Lewis structures show?
The outermost central level of energy-containing electrons is called the level of valence and includes electrons of valence. Lewis symbols are diag...
How do electron dot structures represent shared electrons?
Electron dot structure-valence electrons are represented by dots around the symbol of elements. Electrons sharing — covalent bonding — electrons sh...
What is the purpose of Lewis structures?
The aim of Lewis structures is to provide a simple way for chemists to view molecules that allows accurate predictions about the actual molecules a...
What is the Lewis dot structure in chemistry?
Lewis structures, also known as electron-dot or electron-dot diagrams, are diagrams showing the bonding between a molecule’s atoms and the lone pai...
What is the Lewis structure of ammonia?
Ammonia has the molecular formula NH 3 . It is extremely water-soluble because it is a polar material. For a molecule, the Lewis structure is the t...
Why are Lewis structures important?
For the prediction of geometry, polarity and reactivity of (in)organic compounds, Lewis structures are actually very important. The Lewis structure...
What is a Lewis dot diagram?
A Lewis dot diagram is a simplified representation of a molecule's valence electrons.
Why are Lewis dot diagrams important?
They help us predict a molecule's geometry and how it reacts.
What does VSEPR stand for?
Valence shell electron pair repulsion theory
VSEPR is affected by _____.
Valence electron pairs
What is the effect of lone pairs of electron on the bond angle in VSEPR?
Lone pairs of electron repel other electron pairs more strongly than bonded pairs. This reduces the bond angle.
How to Draw Electron Dot Structures?
A Lewis Electron Dot Formula comprises one dot for every valence electron and an element’s symbol. Stages to articulate the electron dot formula are stated beneath. Note down a skeletal structure displaying a realistic bonding pattern by means of only the element symbols.
What do you call the structure of an electron dot?
Electron dot structures or Lewis dot formula can be drawn if the molecular formula of the compound is known.
How to Draw Lewis Structures?
A Lewis electron dot structure describes the bonding atoms, the number of bonds in the molecule, and the lone pairs left in the bonding atoms.
What is the container that picks up every valence electron?
Pick up every valence electrons from every atom and toss them into a make-believe container that we can term an electron pot.
How is Lewis structure drawn?
The Lewis structure is drawn for individual atoms by putting a dot for each available valence electron around the atom.
How many electrons does carbon monoxide have?
Lewis Structure of CO (Carbon Monoxide) A carbon monoxide molecule consists of one carbon atom and one oxygen atom. The carbon atom requires four electrons to obtain octet configuration whereas the oxygen atom requires two.
How to find the total number of valence electrons in a molecule?
First, the total number of valence electrons present in the molecule is calculated by adding the individual valencies of each atom. If the molecule is an anion, extra electrons (number of electrons added = the magnitude of negative charge) are added to the Lewis dot structure.