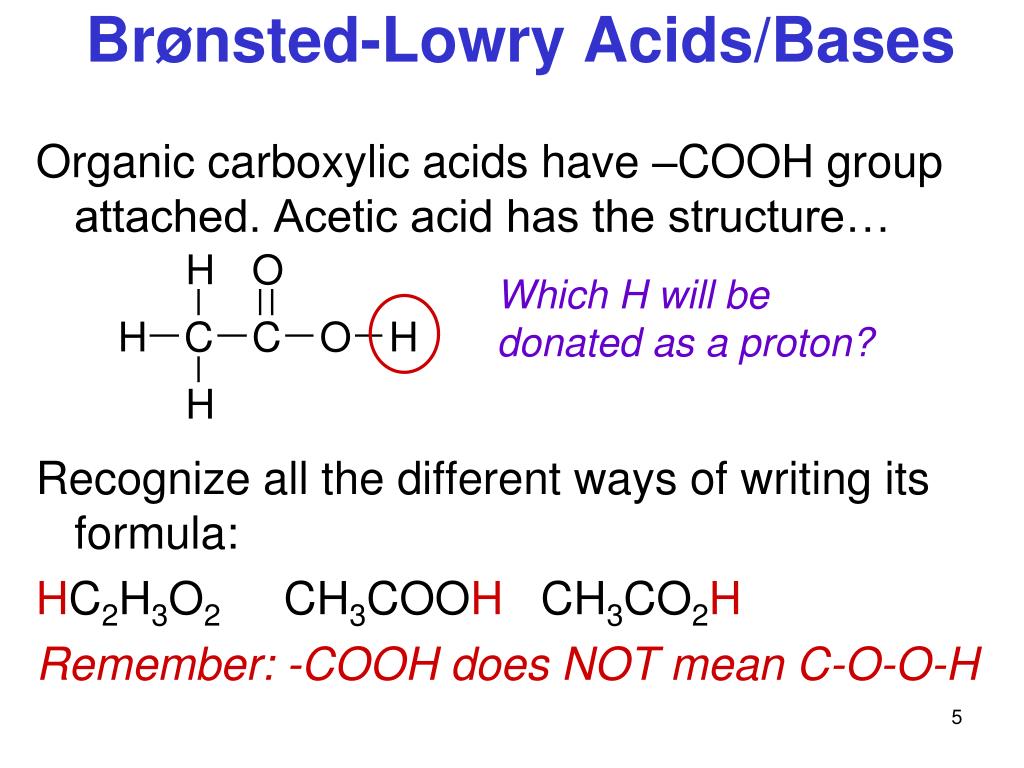
Conjugate acids and conjugate bases are the acids and bases that lose or gain protons. NH4+ is the conjugate acid to the base NH3, because NH3 gained a hydrogen ion to form NH4+.The conjugate base of an acid is formed when the acid donates a proton.
Full Answer
Is NO3 an acid or a base?
Sodium nitrate, NaNO3, is not acidic or basic in the Arrhenius acid-base sense. As an ionic compound, it does contain an anion, but the nitrate ion is on the list of neutral anions. h. Sulfurous acid, H2 SO3, is not on the list of strong acids, so it is a weak acid.
How to find conjugate acid?
How to Find the Conjugate of an Acid
- Steps to Find the Conjugate of an Acid. Step 1: Remove a hydrogen atom from the acid given. ...
- Vocabulary for Finding the Conjugate of an Acid. Acid: A molecule that donates or loses a proton. ...
- Example Problem 1 - Monoprotic acid. What is the conjugate base of the acid, {eq}HNO_3 {/eq}? ...
- Example Problem 2 - Amphiprotic Molecule. ...
What are some examples of acids and bases?
Acids tend to taste sour and feel drying or astringent, while bases taste bitter and feel slippery or soapy. Examples of household acids and bases you can test are vinegar (weak acetic acid) and baking soda solution (diluted sodium bicarbonate -- a base). Acids and bases are important in the human body.
Is HNO3 an acid or base or neutral?
It is a neutral salt because of the neutralization of a strong acid nitric acid (HNO3) with a strong base potassium hydroxide (KOH). This neutralizes each other’s effects and forms a neutral solution whose pH is 7.

What is meant by conjugate acid and conjugate base?
A conjugate acid contains one more H atom and one more + charge than the base that formed it. Conjugate acid is formed when an acid donates a proton to a base. A conjugate base contains one less H atom and one more - charge than the acid that formed it. It is left over substance after acid loses its hydrogen ion.
What is meant by a conjugate base?
conjugate base: substance formed when an acid loses a hydrogen ion. Considered a base because it can gain a hydrogen ion to reform the acid.
What is meant by a conjugate acid?
Conjugate acid-base pair refers to the pair of compounds that differ by a proton. The pair of compounds which can mutually accept and donate hydrogen ions is called a conjugate acid-base pair. A proton is added to obtain the conjugate acid and a proton is removed to get the conjugate base of the compound.
What is the meaning of conjugate in chemistry?
A conjugate acid, within the Brønsted–Lowry acid–base theory, is a chemical compound formed when an acid donates a proton (H +) to a base—in other words, it is a base with a hydrogen ion added to it, as in the reverse reaction it loses a hydrogen ion.
How do you identify conjugate acids and bases?
0:376:03Identify Conjugate Acid Base Pairs (Bronsted Lowry) - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe acid is the one with the extra proton because the acid is the one that can donate the proton.MoreThe acid is the one with the extra proton because the acid is the one that can donate the proton.
Why is it called a conjugate acid?
Whenever an acid donates a proton, the acid changes into a base, and whenever a base accepts a proton, an acid is formed. An acid and a base which differ only by the presence or absence of a proton are called a conjugate acid-base pair.
What is a conjugate example?
What is a conjugate in maths? In maths, Conjugates are defined as a pair of binomials with identical terms but parting opposite arithmetic operators in the middle of these similar terms. For example, p – q is the conjugate of p + q.
How do you find a conjugate acid?
The formula of the conjugate acid is the formula of the base plus one hydrogen ion.
What is the chemical reaction between a conjugate acid and a conjugate base?
The general chemical reaction between a conjugate acid and a conjugate base is: In an acid-base reaction, you can recognize the conjugate base because it is an anion. For hydrochloric acid (HCl), this reaction becomes: Here, the chloride anion, Cl −, is the conjugate base.
What is the chemical reaction of acid and base?
The conjugate acid donates the proton or hydrogen in the reaction. In an acid-base reaction, the chemical reaction is: Acid + Base ⇌ Conjugate Base + Conjugate Acid.
What is the term for the base member of a pair of compounds that transform into each other by gaining or losing?
When an acid dissociates into its ions in water, it loses a hydrogen ion. The species that is formed is the acid's conjugate base. A more general definition is that a conjugate base is the base member, X-, of a pair of compounds that transform into each other by gaining or losing a proton. The conjugate base is able to gain or absorb a proton in a chemical reaction. The conjugate acid donates the proton or hydrogen in the reaction.
What is a conjugate base?
A more general definition is that a conjugate base is the base member, X-, of a pair of compounds that transform into each other by gaining or losing a proton. The conjugate base is able to gain or absorb a proton in a chemical reaction. The conjugate acid donates the proton or hydrogen in the reaction. In an acid-base reaction, the chemical ...
What is the conjugate base of hydrochloric acid?
The conjugate base of hydrochloric acid is the chloride anion. Josh Westrich / Getty Images. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels.
Which species donates hydrogen cation or proton in a reaction?
According to this theory, the species that donates a hydrogen cation or proton in a reaction is a conjugate acid, while the remaining portion or the one that accepts a proton or hydrogen is the conjugate base.