
In contrary to standard oxidation that goes on near the thermodynamic equilibrium, the oxidation in plasma is a non-equilibrium process. Controlled oxidation of organic sulfides to sulfoxides under ambient conditions has been achieved by a series of titanium isopropoxide complexes that use environmentally benign H2O2 as a primary oxidant.
Full Answer
What is a complete oxidation reaction?
Complete oxidation Complete oxidation occurs when the oxygen-to-carbon ratio is at least stoichiometric to produce carbon dioxide and water. The reactions are spontaneous and generate large amounts of energy. Oxidation reactions do not produce hydrogen (refer to Figure 4) and are therefore undesired if hydrogen is the sought-after product.
What is the process of oxidization?
Oxidation occurs when the oxidation state of a molecule, atom or ion is increased. The opposite process is called reduction, which occurs when there is a gain of electrons or the oxidation state of an atom, molecule, or ion decreases. In this reaction, hydrogen is being oxidized and fluorine is being reduced.
What is oxidation and what causes it?
Oxidation occurs when an atom, molecule, or ion loses one or more electrons in a chemical reaction. When oxidation occurs, the oxidation state of the chemical species increases.
What is the opposite of an oxidation reaction?
The opposite process is called reduction, which occurs when there is a gain of electrons or the oxidation state of an atom, molecule, or ion decreases. An example of a reaction is that between hydrogen and fluorine gas to form hydrofluoric acid : H 2 + F 2 → 2 HF In this reaction, hydrogen is being oxidized and fluorine is being reduced.
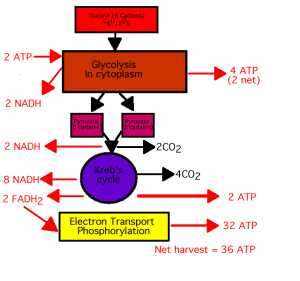
What is a controlled oxidation?
Alkanes on heating with a regulated supply of dioxygen or air at high pressure and in the presence of suitable catalysts give a variety of oxidation products. This process is called controlled oxidation.
What is the benefit of oxidation step in tea processing?
Oxidation is a process through which tea leaves are exposed to the air in order to dry and darken, contributing to the flavor, aroma, and strength of different teas. Just as other fruits and plants, like apples or avocados, brown when exposed to oxygen, tea leaves go through a similar process after they are harvested.
Is oxidized tea good for you?
Oxidation may give black tea nutritional benefits that are not present in green tea. For example, the nutrients in black tea may reduce the risk of several cancers, protect the heart against atherosclerosis, and help maintain healthy blood pressure.
Does oxidation increase caffeine?
One factor that does not impact caffeine level is the level of oxidation. Green, oolong, black and white teas all contain caffeine.
Which tea has the most oxidation?
blackA dark cup and one of the most popular teas is black. This has had the longest oxidation of any tea and is sometimes enjoyed with a splash of milk. Oolong tea is what is produced after an 80% oxidisation and green tea is usually unoxidised to help retain a light fresh taste.
What does fully oxidized mean?
Complete oxidation occurs when the oxygen-to-carbon ratio is at least stoichiometric to produce carbon dioxide and water. The reactions are spontaneous and generate large amounts of energy.
Why is black tea harmful?
Drinking large amounts might cause side effects due to the caffeine content. These side effects can range from mild to serious and include headache and irregular heartbeat. Drinking very high amounts of black tea containing more than 10 grams of caffeine is likely unsafe.
Is coffee a antioxidant?
Antioxidants and Antioxidant Activity. The most antioxidant-rich beverages are [1]: coffee—200–550 mg/cup; tea—150–400 mg/cup; red wine—150–400 mg/glass. Intake of these drinks makes a significant contribution to the total amount of antioxidants consumed by people.
Which is healthier black tea or green tea?
The bottom line. Green and black tea provide similar health benefits, including for your heart and brain. While green tea may contain more powerful antioxidants, the evidence does not strongly favor one tea over the other. Both contain the stimulant caffeine and L-theanine, which has a calming effect.
Is black tea fermented or oxidized?
Oolong tea from Taiwan is grown from the Camellia Sinensis plant. All tea - black tea, green tea, and Oolong tea come from the same plant, and it is largely the degree of oxidation that differentiates them. Green tea is un-oxidized, and black tea is fully oxidized.
How does caffeine increase fat burning?
Caffeine stimulates the nervous system, which sends direct signals to the fat cells, telling them to break down fat (8). It does this by increasing blood levels of the hormone epinephrine ( 9 , 10 ).
Is Matcha an oxidised tea?
As it is a type of green tea, it is also made with non oxidized leaves. Matcha tea is made from shade grown leaves and has a different production process compared to the other three teas. After the leaves are plucked, they are then rolled, and dried in the shade or indoors.
How do you oxidize tea?
The tea leaves must be heated to approximately 150 degrees Fahrenheit to “halt” oxidation. Oxidation is further slowed by drying the leaves, but it never completely stops. At temperatures over 150 degrees Fahrenheit, oxidation continues to occur at an extremely slow pace.
What do oxidative enzymes do?
An oxidative enzyme is an enzyme that catalyses an oxidation reaction. Two most common types of oxidative enzymes are peroxidases, which use hydrogen peroxide, and oxidases, which use molecular oxygen. They increase the rate at which ATP is produced aerobically.
What is meant by oxidation?
Listen to pronunciation. (OK-sih-DAY-shun) A chemical reaction that takes place when a substance comes into contact with oxygen or another oxidizing substance. Examples of oxidation are rust and the brown color on a cut apple.
Is tea fermented or oxidized?
All tea - black tea, green tea, and Oolong tea come from the same plant, and it is largely the degree of oxidation that differentiates them. Green tea is un-oxidized, and black tea is fully oxidized. Oolong tea is in the middle of tea oxidation - usually between 20% and 60% oxidation.
What is the meaning of oxidation?
While the addition of oxygen to a compound typically meets the criteria of electron loss and an increase in the oxidation state , the definition of oxidation was expanded to include other types of chemical reactions.
What is oxidation in chemistry?
Key Takeaways: Oxidation in Chemistry 1 Oxidation occurs when an atom, molecule, or ion loses one or more electrons in a chemical reaction. 2 When oxidation occurs, the oxidation state of the chemical species increases. 3 Oxidation doesn't necessarily involve oxygen! Originally, the term was used when oxygen caused electron loss in a reaction. The modern definition is more general.
What does a rig stand for in oil?
Using OIL RIG to Remember Oxidation and Reduction. So, remember the modern definition of oxidation and reduction concern electrons (not oxygen or hydrogen). One way to remember which species is oxidized and which is reduced is to use OIL RIG. OIL RIG stands for Oxidation Is Loss, Reduction Is Gain.
What is the chemical reaction of rust?
The chemical reaction is: The iron metal is oxidized to form the iron oxide known as rust . Electrochemical reactions are great examples of oxidation reactions. When a copper wire is placed into a solution that contains silver ions, electrons are transferred from the copper metal to the silver ions.
What happens when an atom loses one or more electrons?
Oxidation occurs when an atom, molecule, or ion loses one or more electrons in a chemical reaction. When oxidation occurs, the oxidation state of the chemical species increases. Oxidation doesn't necessarily involve oxygen! Originally, the term was used when oxygen caused electron loss in a reaction. The modern definition is more general.
What is the loss of electrons during a reaction?
Oxidation is the loss of electrons during a reaction by a molecule, atom or ion. Oxidation occurs when the oxidation state of a molecule, atom or ion is increased. The opposite process is called reduction, which occurs when there is a gain of electrons or the oxidation state of an atom, molecule, or ion decreases.
Why is ethanol oxidized?
For example, according to this definition, when ethanol is oxidized into ethanal: CH 3 CH 2 OH → CH 3 CHO. Ethanol is considered oxidized because it loses hydrogen. Reversing the equation, ethanal can be reduced by adding hydrogen to it to form ethanol.
What is complete oxidation?
Complete oxidation occurs when the oxygen-to-carbon ratio is at least stoichiometric to produce carbon dioxide and water. The reactions are spontaneous and generate large amounts of energy. Oxidation reactions do not produce hydrogen (refer to Figure 4) and are therefore undesired if hydrogen is the sought-after product. The complete oxidation of DME is shown in eqn [III]:
Is free energy released in oxidation of organics proportional to EDC?
It has been concluded above that in complete oxidation of organics the free energy released is proportional to the EDC. In biological oxidation of the organics the proportion of this free energy captured by the organism is approximately constant provided the terminal electron acceptor (e.g. oxygen) is the same. This implies that the energy that becomes available to the organism in biological oxidation of organics is proportional to the EDC of the organic.
