What is the function of dynein?
Dynein is a family of cytoskeletal motor proteins that move along microtubules in cells. They convert the chemical energy stored in ATP to mechanical work. Dynein transports various cellular cargos, provides forces and displacements important in mitosis, and drives the beat of eukaryotic cilia and flagella.
Why does dynein have multiple regulatory proteins?
The answer lies in the unique composition of the dynein motor and in the interactions it makes with multiple regulatory proteins that define the time and place where dynein becomes active. Here, we will focus on the different mitotic processes that dynein is involved in, and how its regulatory proteins act to support dynein.
What is the difference between dynein and kinesin?
All of these functions rely on dynein's ability to move towards the minus-end of the microtubules, known as retrograde transport, thus, they are called "minus-end directed motors". In contrast, most kinesin motor proteins move toward the microtubules' plus end.
What is a dynein motor?
Dynein motors are large multisubunit protein complexes divided into two major classes, axonemal dyneins and cytoplasmic dyneins. Cytoplasmic dynein is at the center of several essential functions in eukaryotic cells including cell migration, cell division, maintenance of Golgi integrity, and intracellular transport.

What is dynein associated with?
Dynein and mitochondrial transport Dynein has been implicated as the major motor for microtubule-based retrograde axonal transport, and dynein has been shown to be associated with mitochondria.
Does dynein interact with actin?
Actin motors such as myosin move along microfilaments through interaction with actin, and microtubule motors such as dynein and kinesin move along microtubules through interaction with tubulin.
What cytoskeletal element does dynein interact with?
Dyneins bind to microtubules and move or "walk" from the plus (+) end of the cytoskeletal microtubule filament to the minus (-) end of the filament, which is usually oriented towards the cell center.
What does dynein move on?
Cytoplasmic dynein, a member of the AAA family of ATPases, drives the processive movement of numerous intracellular cargos towards the minus end of microtubules.
What do motor proteins interact with?
Motor proteins have two essential characteristics: they interact with cytoskeletal filaments; and this interaction is modulated as the hydrolysis reaction proceeds, catalyzed by the motor domain.
What are kinesin and dynein associated with?
The motor proteins kinesin and dynein exist to transport biological payloads, such as proteins, organelles, and vesicles, along microtubule pathways, and provide forces to drive motion of flagellar structures and cilia [5].
Is dynein involved in muscle contraction?
The pairs of microtubules are also physically connected via motor proteins called dyneins that move along the microtubules, generating a force that causes the flagellum or cilium to beat.
How do motor proteins and cytoskeletal elements work together?
Perhaps the most fascinating proteins that associate with the cytoskeleton are the molecular motors called motor proteins. These remarkable proteins bind to a polarized cytoskeletal filament and use the energy derived from repeated cycles of ATP hydrolysis to move steadily along it.
How do motor proteins and cytoskeleton work together?
F-actin filaments act as myosin track, while kinesin and cytoplasmic dynein move on microtubules. Cytoskeleton motors work together to build a highly polarized and regulated system in neuronal cells via different molecular mechanisms and functional regulations.
Does dynein move towards the nucleus?
At the nuclear envelope, dynein is thought to walk along microtubules towards their minus-ends. Since the microtubule minus-ends are embedded in the centrosome, minus-end directed motility of dynein results in a pulling force that brings the nucleus and centrosome towards each other.
What is the function of the dynein arms?
The outer dynein arm (ODA) is a molecular complex that drives the beating motion of cilia/flagella.
Does dynein use ATP?
Dyneins are large molecular motors that hydrolyze ATP to generate a minus-end-directed force along microtubules. Each dynein consists of one to three dynein heavy chains (HCs), which encompass the ATPase activity, complexed to intermediate (IC), light-intermediate (LIC), and light chains (LC).
Does dynein require ATP?
Thus, ATP binding to AAA4 is required for dynein motility. Fig. 2: AAA4-ATP binding is essential for dynein motility.
Can dynein break down ATP?
Evolutionary Biology of Dyneins Dyneins are large molecular motors that hydrolyze ATP to generate a minus-end-directed force along microtubules.
What is Dynactin used for?
Dynactin is a multi-subunit complex that binds to dynein and the kinesin-2 subset of kinesins (Kardon and Vale, 2009), where it links motors to organelle or vesicle cargo, is often essential for motor activity and enhances motor processivity.
What is the function of Axoneme?
The axoneme serves as the "skeleton" of these organelles, both giving support to the structure and, in some cases, the ability to bend. Though distinctions of function and length may be made between cilia and flagella, the internal structure of the axoneme is common to both.
What is a dynein complex?
Dynein complexes are composed of one to three heavy chains, and each complex also has various smaller accessory subunits (Tables 1 and 2 ). Dyneins are classified as either cytoplasmic or axonemal. The green alga, Chlamydomonas, which has two flagella, has been a useful model system for studies of axonemal dyneins.
What are the two classes of dyneins?
Dyneins are evolutionarily distinct from the two other families of cytoskeletal motors, myosins and kinesins, and are organized into two classes: axonemal and cytoplasmic. From: Encyclopedia of Cell Biology, 2016. Download as PDF. About this page.
How many chains does a dynein have?
Dynein complexes are composed of one, two, or three heavy chains, and each complex has various smaller accessory subunits ( Tables I and II). Cytoplasmic dynein has two identical heavy chains. Flagellar outer arm dyneins have two or three different heavy chains depending on the species.
What are the arms that project from the doublet axonemal microtubules of the flagella?
The flagellar dyneins , also called axonemal dyneins, are the arms that project from the doublet axonemal microtubules of flagella and cilia. The flagellar dyneins generate the sliding force between outer doublet microtubules that is converted by other axonemal structures into the bending of cilia and flagella.
How many flagellar dynein heavy chain genes are there?
Molecular genetic analyses indicate that genomes contain more than ten flagellar dynein heavy chain genes, one cytoplasmic dynein heavy chain gene, and the intraflagellar transport dynein heavy chain, which is most related to the cytoplasmic dynein heavy chain.
Which type of cytoplasmic dynein is responsible for the transport of proteins from the tips of?
The second type of cytoplasmic dynein, cytoplasmic dynein 2, is responsible for a component of intraflagellar transport (IFT), specifically the transport of protein complexes from the tips of cilia and flagella to their base, that occurs between the axonemal microtubules and the flagellar membrane.
Which model proposes that dyneins on one side of the axoneme are active and?
The Switching model ( Satir and Matsuoka, 1989; Wais-Steider and Satir, 1979) proposed that dyneins on one side of the axoneme is active and the other side is inactive, depending on the curvature. Recent cryo-ET of sperm flagella supports this model ( Lin et al., 2014 ).
What is a dynein?
Dyneins are large molecular motors that hydrolyze ATP to generate a minus-end-directed force along microtubules. Each dynein consists of one to three dynein heavy chains (HCs), which encompass the ATPase activity, complexed to intermediate (IC), light-intermediate (LIC), and light chains (LC).
What are dyneins in plants?
Dyneins are found in many eukaryotes, including fungi, worms, insects, and vertebrates, but genome sequence analyses indicate that they are not present in flowering plants. Dynein complexes are composed of one to three heavy chains, and each complex also has various smaller accessory subunits (Tables 1 and 2 ). Dyneins are classified as either cytoplasmic or axonemal. The green alga, Chlamydomonas, which has two flagella, has been a useful model system for studies of axonemal dyneins. These dyneins are the arms which project from the doublet axonemal microtubules of flagella and cilia. The axonemal dyneins generate the sliding force between outer doublet microtubules that is converted by other axonemal structures into the bending of cilia and flagella. The axonemal dyneins are further divided into the outer arm and inner arm dyneins defined by their position of the outer doublet microtubules. Of the more than ten different dynein complexes identified in animals, most are components of the flagellar axoneme. Various organisms, from yeast to mouse, have been utilized for studies on cytoplasmic dynein. Cytoplasmic dynein 1 moves membranous organelles, kinetochores, and viruses along microtubules. It is also the motor for retrograde axonal transport and is involved in the assembly and function of the mitotic spindle. The second type of cytoplasmic dynein, cytoplasmic dynein 2, is responsible for a component of intraflagellar transport (IFT), specifically the transport of protein complexes from the tips of cilia and flagella to their base, that occurs between the axonemal microtubules and the flagellar membrane. This dynein is often referred to as IFT dynein.
What is the function of dynein in a glass coverlip?
Dynein is attached to a glass coverslip and when microtubules are added, the dynein motor domains bind the microtubules. In the presence of MgATP, the dynein moves microtubules across the coverslip with the microtubule-plus ends leading.
How much force does dynein produce?
Dynein’s force production has also been measured. In vitro, our lab measures purified bovine dynein to stall at approximately 1.1–1.2 pN of force [9–11], the same as for murine [5] and rat dyneins (J. Xu and S. P. Gross, unpublished work). The ∼1.1 pN stall force has also been measured for murine dynein by the Holzbaur group (personal communication) and for Dictystelium dynein by the Mallik Group [12]. However, yeast dynein is reported to stall at 5–7 pN [13]. Interestingly, the Higuchi group reports a stall force of 5–7 pN for bovine dynein [14], which they attribute to the details of how the dynein is attached to the cargo. This seems unlikely to us, since our group received their protocol but was unable to reproduce their findings. The overall cause of this discrepancy is unknown, but we have suggested it may reflect kinesin contamination [11]. The bona fide differences between bovine dynein and yeast dynein may reflect cellular specialization, because yeast does not use dynein/microtubule-based transport for vesicular transport but rather for slowly moving/repositioning large cargos such as the nucleus. Consistent with such specialization, yeast dynein is very slow, moving at ∼50–80 nm/s.
What is the function of axonemal dyneins?
The axonemal dyneins generate the sliding force between outer doublet microtubules that is converted by other axonemal structures into the bending of cilia and flagella. The axonemal dyneins are further divided into the outer arm and inner arm dyneins defined by their position of the outer doublet microtubules.
How fast does dynein move?
In summary, by itself at the single-molecule level in vitro, dynein moves at approximately 800 nm/s, with approximately one μm of mean travel (with the exception of murine dynein [5] ). It can move against loads of up to 1.2 pN. Note that these stated velocity and travel distances reflect “unloaded” dynein – when moving a cargo against a load, dynein moves slower and is more prone to detachment from the microtubule. The larger the opposing load, the slower the motion and the shorter the mean travel.
What is the cytoplasmic dynein complex?
Cytoplasmic dynein 1 moves membranous organelles, kinetochores, and viruses along microtubules.
What is the role of dynein in cell division?
Furthermore, dynein plays essential roles during cell division where it is implicated in multiple processes including centrosome separation, chromosome movements, spindle organization, spindle positioning, and mitotic checkpoint silencing. How is a single motor able to fulfill this large array of functions and how are these activities temporally ...
What is a cytoplasmic dynein?
Cytoplasmic dynein is a large minus-end-directed microtubule motor complex, involved in many different cellular processes including intracellular trafficking, organelle positioning, and microtubule organization. Furthermore, dynein plays essential roles during cell division where it is implicated in multiple processes including centrosome separation, chromosome movements, spindle organization, spindle positioning, and mitotic checkpoint silencing. How is a single motor able to fulfill this large array of functions and how are these activities temporally and spatially regulated? The answer lies in the unique composition of the dynein motor and in the interactions it makes with multiple regulatory proteins that define the time and place where dynein becomes active. Here, we will focus on the different mitotic processes that dynein is involved in, and how its regulatory proteins act to support dynein. Although dynein is highly conserved amongst eukaryotes (with the exception of plants), there is significant variability in the cellular processes that depend on dynein in different species. In this review, we concentrate on the functions of cytoplasmic dynein in mammals but will also refer to data obtained in other model organisms that have contributed to our understanding of dynein function in higher eukaryotes.
Making a molecular motor fit for purpose
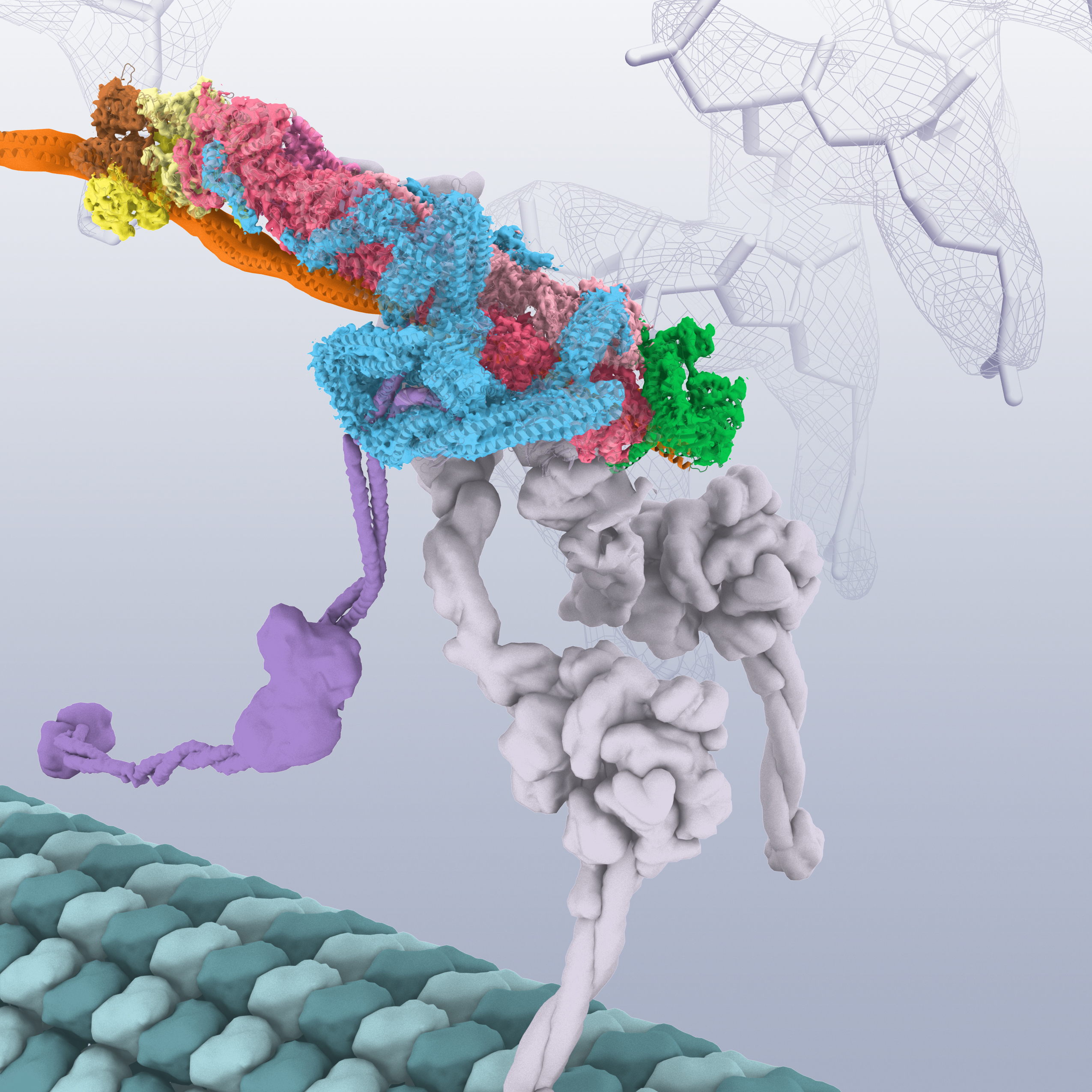
Dynactin is an essential cofactor of the microtubule motor, cytoplasmic dynein. Dynactin contains 23 subunits built around a short filament of an actin-related protein (Arp1). How dynactin is assembled, how it functions with dynein, and why it is built around an actin-like filament is unclear. Urnavicius et al.
Abstract
Dynactin is an essential cofactor for the microtubule motor cytoplasmic dynein-1. We report the structure of the 23-subunit dynactin complex by cryo-electron microscopy to 4.0 angstroms. Our reconstruction reveals how dynactin is built around a filament containing eight copies of the actin-related protein Arp1 and one of β-actin.
Dynactin structure determination
Dynactin is a challenging target for cryo-EM ( 17 ). This complex’s extreme preferred orientation on EM grids makes it hard to obtain the broad distribution of views required for a three-dimensional (3D) reconstruction. Furthermore, dynactin’s thin elongated shape limits its contrast, making it difficult to assign views accurately.
The dynactin filament contains eight Arp-1 subunits and one β-actin
The dynactin filament is nine subunits long and consists of two protofilaments that wrap around each other ( Fig. 1, A and C ): five subunits (A, C, E, G, and I) in the top protofilament and four (B, D, F, and H) in the bottom. The presence of β-actin in the filament is controversial ( 7, 23 ). Our cryo-EM map was of sufficient quality (fig.
Capping the dynactin filament
The dynactin filament is similar to that of actin ( 24 ), consistent with the high (53%) sequence identity between β-actin and Arp1 (fig. S3B). Both consist of four subdomains surrounding a nucleotide binding site (fig. S4).
The p150 Glued projection extends more than 50 nm from the shoulder
Previous antibody labeling showed that p150 Glued forms dynactin’s shoulder projection ( 7 ). Existing models suggest that it is 24 nm long and contains the p150 Glued N-terminal Cap-Gly domain and the CC1A coiled coil ( Fig. 3A) ( 7, 8 ). Owing to its flexibility, the projection is not visible in our high-resolution EM maps.
Extended peptides from the shoulder span the length of the dynactin filament
The invariant size of the dynactin filament implies that some mechanism specifies its length ( 7, 17, 32 ). The shoulder is the best candidate for dynactin’s molecular ruler. Its main body contacts four Arp1 subunits close to the barbed end (fig. S10).
Where is Dynein found in the cell?
Dynein is a motor protein present in the cytoskeleton of the cells. They are capable of moving along microtubules and aiding the intracellular transport of vesicles and organelles. Dynein travels towards the minus end of the microtubules. While traveling, they carry materials towards the center of the cell since the minus end is towards ...
What is the Difference Between Dynein and Kinesin?
The key difference between dynein and kinesin is the direction of their movement along the microtubules. Dynein moves towards the minus end of the microtubule while kinesin moves towards the plus end of the microtubule. Moreover, dynein transports cargo from the periphery to the center of the cell while kinesin transports cargo from the center to the periphery of the cell. Thus, this is an important functional difference between dynein and kinesin. Structurally, dynein protein consists of one to three heavy chains complex to intermediate, light-intermediate and light chains. On the other hand, kinesin protein consists of two heavy chains and two light chains. Therefore, this is another difference between dynein and kinesin.
What is Kinesin?
Kinesin is another type of cytoskeleton motor protein that is capable of moving along the microtubules filaments. Moreover, kinesins are ATPases. Their movements are energy consuming. Most kinesins travel towards the plus end of the microtubules that are present towards the periphery of the cell (towards the cell surface). While traveling, kinesins carry cargo (organelles and vesicles) from the center of the cell to the periphery of the cell (anterograde transport).
What are the cargoes that kinesins carry?
While traveling, kinesins carry cargo (organelles and vesicles) from the center of the cell to the periphery of the cell (anterograde transport). Figure 02: Kinesin. Mutations of kinesin proteins can lead to nervous system disorders. One such common disease is peripheral neuropathy.
What is Dr. Samanthi Udayangani's degree?
Dr.Samanthi Udayangani holds a B.Sc. Degree in Plant Science, M.Sc. in Molecular and Applied Microbiology, and PhD in Applied Microbiology. Her research interests include Bio-fertilizers, Plant-Microbe Interactions, Molecular Microbiology, Soil Fungi, and Fungal Ecology.
Which two proteins move along the microtubules?
Both dynein and kinesin move along the microtubules.
Who provided antibodies to kinesin and dynein?
We gratefully acknowledge the contributions of Spencer Shelly and Penney Gilbert of the University of Pennsylvania. We also thank Scott Brady, Richard Vallee, and Stephen King, who provided antibodies to kinesin and dynein, and Larry Goldstein, who provided cDNA clones of KLCs 1 and 2.
Is cytoplasmic dynein mediated by kinesin?
Together, these observations identify a direct interaction between cytoplasmic dynein and kinesin, mediated by kinesin light chains. Although light chains may not be necessary for all kinesin-cargo interactions, some motor-cargo interactions appear to be mediated by direct binding to KLCs (