How do you write an orbital box diagram?
An orbital box diagram can be written as well. Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin–one arrow pointing up and the other one pointing down. The orbital box diagrams are listed for the first 20 elements in the figure below.
What do the arrows mean on an orbital box diagram?
Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin–one arrow pointing up and the other one pointing down. The orbital box diagrams are listed for the first 20 elements in the figure below.
What is the electron configuration for carbon in a box diagram?
The electron configuration for carbon is 1s22s22p2. An orbital box diagram can be written as well. Boxes, or horizontal lines represent the orbitals, arrows represent the electrons, and if an orbital is full, the electrons must be of opposite spin–one arrow pointing up and the other one pointing down.
What symbol do you use to report the orbital diagram?
In your response, use the elemental symbol. The orbital diagram provided represents what element? Report your answer using the chemical symbol for the element. Nice work! You just studied 13 terms!
What does each orbital represent?
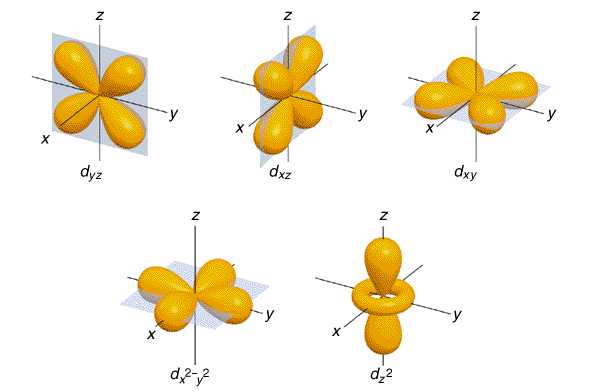
Atomic orbitals are commonly designated by a combination of numerals and letters that represent specific properties of the electrons associated with the orbitals—for example, 1s, 2p, 3d, 4f. The numerals, called principal quantum numbers, indicate energy levels as well as relative distance from the nucleus.
What are the boxes in electron configuration?
The boxes are used to represent the orbitals and to show the electrons placed in them. The order of fill is the same but as you can see from above the electrons are placed singly into the boxes before filling them with both electrons.
What are the 4 orbitals describe each?
There are four basic types of orbitals: s, p, d, and f. An s orbital has a spherical shape and can hold two electrons. There are three p orbitals, each of which has the same basic dumbbell shape but differ in its orientation in space. The p orbitals can hold up to six electrons.
What is 1s 2s 2p 3s 3p?
1s 2s 2p 3s 3p represents the electron orbital energy levels.
How do you write Box notation?
1:334:55How to write orbital notation - YouTubeYouTubeStart of suggested clipEnd of suggested clipAny time I hit a D I'm gonna draw five boxes. And if I were hitting an F. I would draw seven boxesMoreAny time I hit a D I'm gonna draw five boxes. And if I were hitting an F. I would draw seven boxes these correspond to how many orbitals s P D.
What are the 4 orbitals and how many electrons?
Orbitals and Electron Capacity of the First Four Principle Energy LevelsPrinciple energy level (n)Type of sublevelMaximum number of electrons (2n2)4s32pd7 more rows
What are the 4 Subshells?
Within the shells, electrons are further grouped into subshells of four different types, identified as s, p, d, and f in order of increasing energy. The first shell has only an s subshell; the second shell has an s and a p subshell; the third shell has s, p, and d subshells, and the fourth has s, p, d and f subshells.
How orbitals are arranged in atom?
The orbitals in an atom are arranged according to energy levels, and in these energy levels the orbitals are ordered s, p, d, and f. And the higher the energy level, the more orbitals it has.
How do you draw an electron box?
6:2612:54Electron Box Diagrams - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo we're going to add electrons to this box diagram. And instead of using dots like we did in bohr'sMoreSo we're going to add electrons to this box diagram. And instead of using dots like we did in bohr's model we're going to use arrows or what i use is half arrows to re represent the electrons.
How do you read electron configuration?
0:023:32Using the Electron Configuration Chart - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe first thing we need to do to find electron configurations is to find the number of electrons forMoreThe first thing we need to do to find electron configurations is to find the number of electrons for the element. So we can find this on the periodic. Table and we're going to look at the number above
How do you draw an electron box diagram?
1:284:37ALEKS: Drawing a box diagram of the electron configuration of an atomYouTubeStart of suggested clipEnd of suggested clipEach there are three 2p orbitals and there are three 3p orbitals. So what we're going to do is drawMoreEach there are three 2p orbitals and there are three 3p orbitals. So what we're going to do is draw a box or use alex to draw a box to represent. Each one of these different shells.
Which orbital is singly occupied before any orbital is doubly occupied?
1. Every orbital in a sublevel is singly occupied before any orbital is doubly occupied.
How many electrons can be in a single orbital?
that a maximum of two electrons may occupy a single atomic orbital, but only if the electrons have opposite spins.
Which electrons have the elctron configuration of the nearest noble gas element?
Inner shell electrons that have the elctron configuration of the nearest noble-gas element
How many pairs of electrons are in a single atom?
Localized on a single atom and contains one pair of nonbonding electrons ( a lone pair)
How many electron domains are there in an ion?
The number of electron domains in a molecule or ion is the number if bonds (double and triple bonds count as one domain) plus the number of nonbonding (lone) electron pairs.
What are the 3s and 3p electrons?
The 3s and 3p electrons are valence electrons.
What does each half arrow represent?
Each half arrow in an orbital diagram represents an electron. The direction of the half-arrow represents electron spin.
What is energy released in a spontaneous redox reaction?
Energy released in a spontaneous Redox reaction is used to preform work by the transfer of e-'s
