What is an ionic bond most likely to form between?
An ionic bond is most likely to form between metal and nonmetal elements. An element can be classified as a metal if it’s found on the left side of the periodic table, while nonmetals are found on the right side of the periodic table.
What are two things do ionic bonds form between?
Key Points
- Ionic bonds are formed between cations and anions.
- A cation is formed when a metal ion loses a valence electron while an anion is formed when a non-metal gains a valence electron. ...
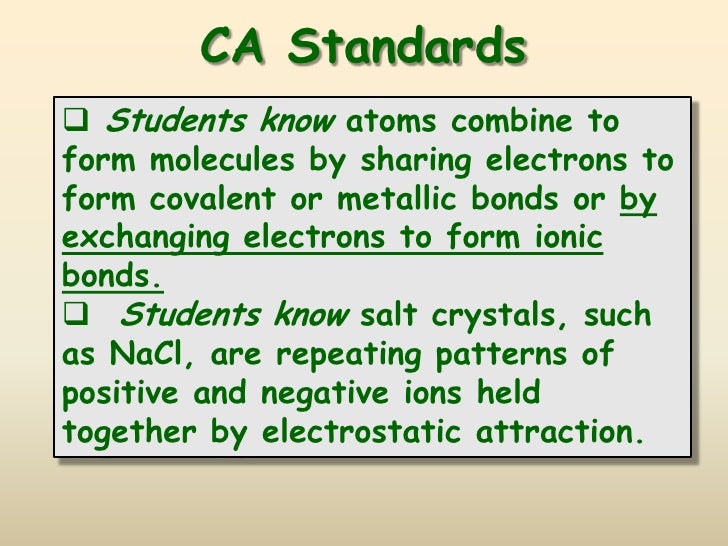
- Ionic solids form crystalline lattices, or repeating patterns of atoms, with high melting points, and are typically soluble in water.
What is the difference between covalent and ionic bonding?
The main difference between covalent and ionic bonds is that ionic bonds occur between two species which are electrostatically attracted towards each other, whereas covalent bonds occur covalently through the sharing of electrons between their outer shells. In general, metallic elements tend to form ionic bonds, and non-metallic elements tend to form covalent bonds.
What type of atoms do ionic bonds occur between?
An ionic bond is a force of attraction between a positive and a negative charged ion. These oppositely charged ions are usually produced when a metal transfers its electron to a nonmetal. For example, when an electron is removed from a neutral sodium atom, the sodium atom becomes a positively charged sodium ion (Na + ), and when this electron ...

Are ionic bonds between metals and nonmetals?
In general, covalent bonds form between nonmetals, ionic bonds form between metals and nonmetals, and metallic bonds form between metals.
Where do ionic bonds always occur?
Ionic bonding always occurs between positively charged ions, called cations, and negatively charged ions, called anions. In both cases, the ions have the electron configuration of a noble gas. One element loses electrons.
Where do the bonds occur?
Bonds form when atoms share or transfer valence electrons. Valence electrons are the electrons in the outer energy level of an atom that may be involved in chemical interactions. Valence electrons are the basis of all chemical bonds.
Where are ionic bonds found in a cell?
You would be most likely to find ionic bonds in proteins. Proteins consist from long strings of amino acids held together by peptide linkages. Some of these amino acids have acidic side-chains ( aspartic and glutamic acid).
What is an ionic bond? Explain with an example?
When a positively charged ion forms a bond with a negatively charged ion, one atom donates electrons to the other, this is known as an ionic bond....
What kind of force is present in ionic bonds?
Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. For both atoms involved, this...
How do you find ionic formulas?
To obtain an ionic compound’s formula, first determine the cation and record its symbol and charge. Then, write down the anion’s symbol and charge...
Are ionic bonds between two nonmetals?
Consider whether each element is a metal or a nonmetal to anticipate the sort of bond that will form between them. In general, nonmetals form coval...
How do you break ionic bonds?
Ionic chemicals dissolve in polar solvents like water because they are polar. When polar solvents disrupt the ionic bonds, they dissolve. By dissol...
What is a covalent bond?
A covalent bond is formed by the sharing of electrons between participating atoms. In it, each atom contributes an equal number of electrons for bo...
Can an ionic bond form between two metals?
No, the ionic bond can not form between two metals. An ionic bond is formed between positive and negative ions or a metal and a non-metal by the do...
How are ionic bonds formed between atoms?
Ionic bonds are formed by the complete transfer of electrons between two atoms. An atom that gains an electron acquires a negative charge and is kn...
Do ionic bonds have a high melting point?
Yes, ionic bonds have a high melting point. Ionic bonds are formed by the complete transfer of electrons held together by a strong electrostatic fo...
What is an Ionic Bond?
The electrostatic force of attraction which holds the two oppositely charged ions together is called the ionic bond.
What is the difference between ionic and covalent bonds?
The ionic bond is the attraction between positive and negative ions in a crystal and compounds held together by ionic bonds are called ionic compounds. The covalent bond is a bond formed when two atoms share one or more electron pairs. Each atom contributes an equal number of electrons towards the bond formation.
What type of bond is formed by the complete transfer of valence electrons to attain stability?
An Ionic bond is the bond formed by the complete transfer of valence electron to attain stability. This type of bonding leads to the formation of two oppositely charged ions – positive ion known as cations and negative ions are known as anions. The presence of two oppositely charged ions results in a strong attractive force between them.
What type of bond is formed when one atom gains electrons while the other atom loses electrons from its?
The bond formed by this kind of combination is known as an ionic bond or electrovalent bond. This kind of bond is formed when one atom gains electrons while the other atom loses electrons from its outermost level or orbit.
What is the force between two oppositely charged ions?
The presence of two oppositely charged ions results in a strong attractive force between them. This force is an ionic or electrovalent bond. Ionic bonds form between atoms with large differences in electronegativity, whereas covalent bonds formed between atoms with smaller differences in electronegativity.
What type of bond does each element have?
Consider whether each element is a metal or a nonmetal to anticipate the sort of bond that will form between them. In general, nonmetals form covalent bonds, metals and nonmetals create ionic connections, and metals and metals create metallic connections.
What is the term for the transfer of electrons between atoms?
Electrovalent Bond . Electrovalent bonds are produced when electrons are transferred from atoms of one element to atoms of another element, producing positive and negative ions. The bond which is formed by the transfer of electrons between the atoms is called electrovalent bond or ionic bond.
What is an Ionic Bond?
An ionic bond is a type of chemical bond formed by electrostatic attraction between two oppositely-charged ions. These ions are created by the transfer of valence electrons between two atoms, usually a metal and a non-metal.
How Are Ionic Bonds Formed?
Oppositely-charged ions have a strong electrostatic attraction between them. This attraction is the ionic bond, and it allows a positive ion and a negative ion to form a stable ionic compound with a neutral charge.
How Are Ions Created?
Ions are created when an atom loses or gains an electron. An atom that gains an electron becomes negatively charged, and is called an anion. An atom that loses an electron becomes a positively-charged cation.
Why are ionic bonds stronger than covalent bonds?
Ionic bonds are generally stronger than covalent bonds because of the electrostatic attraction that exists between oppositely charged ions.
What happens to the atoms in an ionic bond?
During ionic bonding, two atoms (usually a metal and a non-metal) exchange valence electrons. One atom acts as an electron donor, and the other as an electron acceptor. This process is called electron transfer and creates two oppositely-charged ions.
Why do ionic compounds shatter?
It takes a lot of force to break the ionic bonds that hold them together but, if enough force is applied, they shatter easily. This happens because breaking the ionic bonds brings ions of the same charge together.
What type of bond is formed when ions bind together?
Ionic bonds are one of the two main types of chemical bonds. They form as a result of electrostatic attraction between oppositely charged ions and usually occur between metals and non-metals. When lots of ions bind together, they form a giant, regular, 3D structure called the ionic lattice, or crystal lattice.
What happens to the metal atoms in a bond?
In all of these reactions, the metal atoms give electrons to the non-metal atoms. The metal atoms become positive ions and the non-metal atoms become negative ions. There is a strong electrostatic force of attraction between these oppositely charged ions – this is called an ionic bond.
What is the bonding process that occurs when metals react with non-metals?
Ionic bonding. When metals react with non-metals, electrons are transferred from the metal atoms to the non-metal atoms, forming ions. The resulting compound is called an ionic compound.
When a metal element reacts with a non-metal element, an ionic compound is formed?
When a metal element reacts with a non-metal element an ionic compound is formed. When a non-metal element reacts with a non-metal element a covalent bond is formed. An understanding of the way the elements are bonded allows us to explain their typical properties.
