Does equilibrium lie to the left or right?
Yes, "to the left" refers to the left side of an equilibrium expression. When we talk about equilibrium lying "to the left", it means that the educt/reactant is favored, i.e. more than or . Conversely, an equilibrium that lies "to the right" is one that favors products.
What happens to the position of equilibrium when temperature increases?
If the temperature is increased, then the position of equilibrium will move so that the temperature is reduced again. The position of equilibrium therefore moves to the left. Decreasing the temperature?
What does equilibrium rely on?
It basically rely on the fact what a question has been asked for particles ions or atoms. Now to see, we need to first see is there more particles on the left hand side or the right hand side. Where there are more particles, equilibrium rely that that hand or side, so the forward or the backward reaction.
Which way does the position of equilibrium move in a reaction?
The equilibrium will move in such a way that the pressure increases again. It can do that by producing more gaseous molecules. In this case, the position of equilibrium will move towards the left-hand side of the reaction.
What equilibrium lies to the right?
What does "to the left" mean in math?
About this website

Chem 2 Exam 3 Chapter 15 MC Flashcards | Quizlet
Start studying Chem 2 Exam 3 Chapter 15 MC. Learn vocabulary, terms, and more with flashcards, games, and other study tools.
CHEM 1172 EXAM 2 [CH 13+14] Flashcards | Quizlet
Study with Quizlet and memorize flashcards containing terms like 1. The reaction A + 2B products has the rate law, rate = k[A][B]3. If the concentration of B is doubled while that of A is unchanged, by what factor will the rate of reaction increase? A) 2 B) 4 C) 6 D) 8 E) 9, 2. Appropriate units for a second-order rate constant are A) M/s B) 1/Mls C) 1/s D) 1/M2ls, 3.
Solved 1. A 0.10 M NH3 solution is 2.5% ionized. Calculate | Chegg.com
Answer to Solved 1. A 0.10 M NH3 solution is 2.5% ionized. Calculate. Science; Chemistry; Chemistry questions and answers; 1. A 0.10 M NH3 solution is 2.5% ionized.
Which side of the reaction does equilibrium move?
It can do that by producing more gaseous molecules. In this case, the position of equilibrium will move towards the left-hand side of the reaction.
What happens when a chemical reaction is at equilibrium?
If a chemical reaction is at equilibrium and experiences a change in pressure, temperature, or concentration of products or reactants, the equilibrium shifts in the opposite direction to offset the change.
What happens to equilibrium when temperature is increased?
Increasing the temperature. If the temperature is increased, then the position of equilibrium will move so that the temperature is reduced again. The position of equilibrium therefore moves to the left.
What happens when the concentration of A decreases?
Decreasing a concentration. In the opposite case in which the concentration of A is decreased, according to Le Châtelier, the position of equilibrium will move so that the concentration of A increases again.
What happens to the pressure of a container when the pressure is increased?
According to Le Châtelier, if the pressure is increased, the position of equilibrium will move so that the pressure is reduced again. The more molecules in the container, the higher the pressure will be. The system can reduce the pressure by reacting in such a way as to produce fewer molecules.
Does adding a catalyst affect equilibrium?
This is because a catalyst speeds up the forward and back reaction to the same extent and adding a catalyst does not affect the relative rates of the two reactions, it cannot affect the position of equilibrium.
Does increasing the pressure affect the equilibrium?
In this case, increasing the pressure has no effect on the position of the equilibrium. Because there are equal numbers of molecules on both sides, the equilibrium cannot move in any way that will reduce the pressure again.
What does it mean when equilibrium is to the right?
If K > 1, the position of equilibrium lies to the right, meaning the formation of the products is favored in the reaction.
What is the state of equilibrium?
Dynamic equilibrium, or chemical equilibrium, refers to the state a chemical reaction is in when the forward and reverse reactions are at equal rates, meaning that the concentrations of products and reactants both remain constant. The rate in which products are being formed from the reactants is the same as the rate where products are being broken back down into reactants.
What is the equilibrium constant? What does it represent?
The equilibrium constant, K, represents the extent of a reaction when it is at equilibrium. It uses the concentrations and coefficients of each reactant and product to form a ratio. From the K value, we can understand whether a reaction favors the reactants or products more, and therefore where the position of equilibrium lies.
What is the reaction quotient?
The reaction quotient is a measure of the extent of reaction at any given moment, not solely at equilibrium. This means that it takes into account the relative amounts (concentrations) of reactants and products at that moment in time. In this vein, Q = [C]c[D]d / [A]a[B]b, with the concentrations of reactants and products representing their actual concentrations at the given time.
How to find equilibrium constant?
For an equilibrium equation aA + bB ⇌ cC + dD, the equilibrium constant, can be found using the formula K = [C]c[D]d / [A]a[B]b , where K is a constant. The concentrations of all the products are in the numerator and the concentrations of all the reactants are in the denominator; each component is then raised to the power of their specific coefficient.
Why is it important to keep the expression of concentrations of all solutions consistent?
This is very important because K is found using the concentrations of products and reactants. Most commonly, solutions are expressed in M, or occasionally in moles if all solutions are in the same volume. Solids, liquids, and solvents are assigned a value of 1, so their concentrations do not affect the value of K. In this manner, only aqueous solutions and gases count towards the equilibrium constant expression.
When adding 2 or more reactions together to create a new one, multiply all the existing K values together?
When adding 2 or more reactions together to create a new one, multiply all the existing K values together to obtain the equilibrium constant for the new reaction. K new = K 1 x K 2 x K 3 x …
What equilibrium lies to the right?
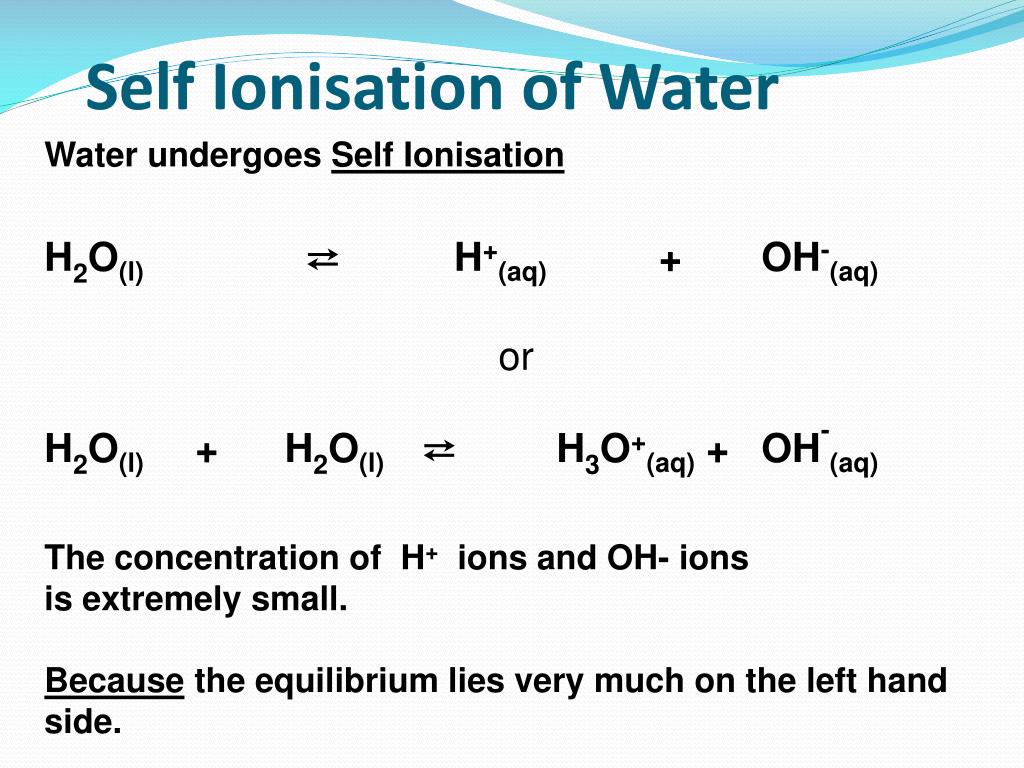
Conversely, an equilibrium that lies "to the right" is one that favors products. In this case, it would favor the production of H X 3 O X + and O H X − over H X 2 O,
What does "to the left" mean in math?
Yes, "to the left" refers to the left side of an equilibrium expression.