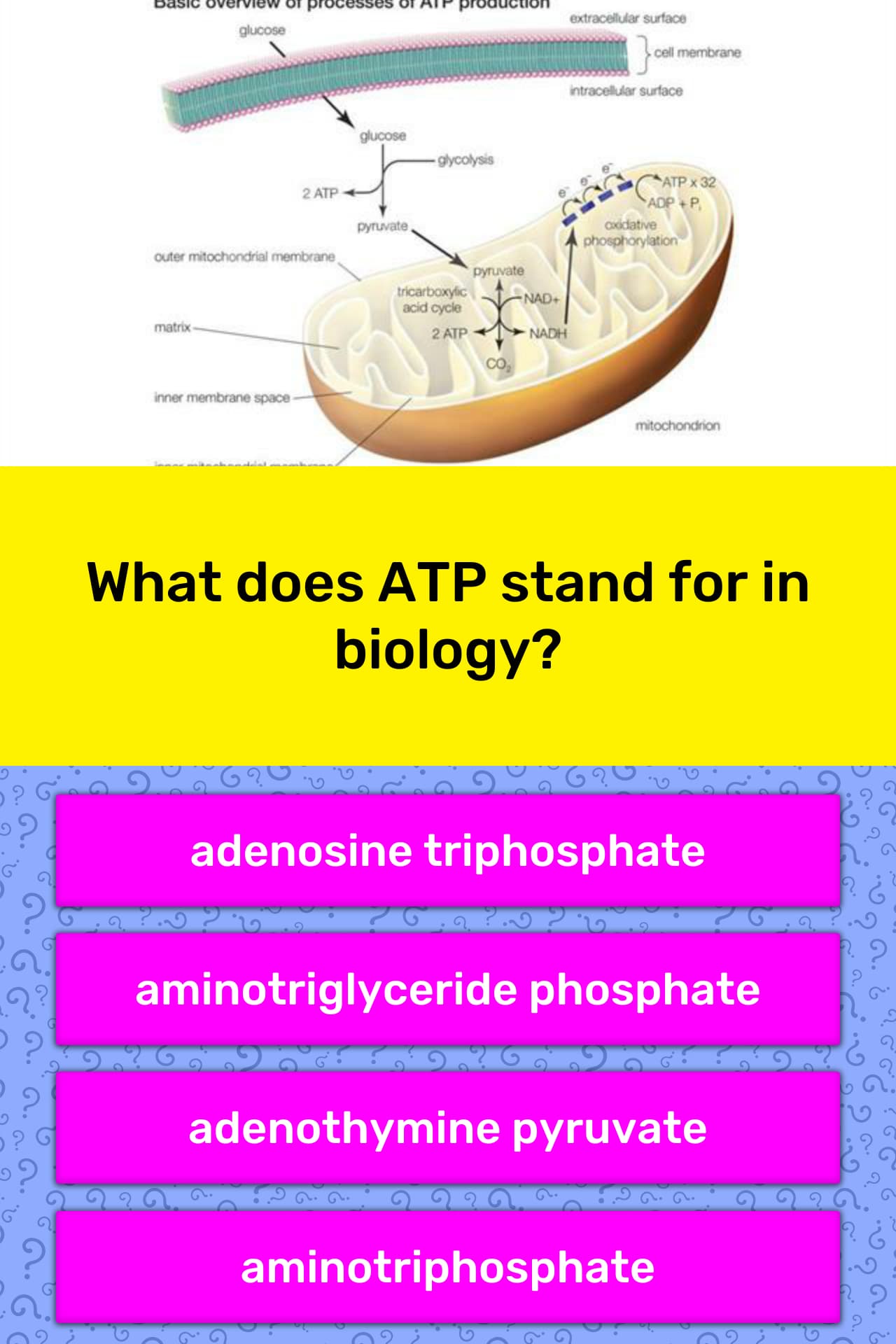
What does oxidative mean in oxidative phosphorylation? Medical Definition of oxidative phosphorylation : the synthesis of ATP by phosphorylation of ADP for which energy is obtained by electron transport and which takes place in the mitochondria during aerobic respiration.
What is oxidative phosphorylation and what is its purpose?
Herein, what is oxidative phosphorylation and where does it occur? Oxidative phosphorylation is a mechanism for ATP synthesis in both plant and animal cells. It involves the chemiosmotic coupling of electron transport and ATP synthesis. Oxidative phosphorylation occurs in the mitochondria. The mitochondrion has two membranes: an inner membrane and an outer …
What is oxidative phosphorylation and where does it occur?
Oxidative Phosphorylation Definition “Oxidative phosphorylation is the process of ATP formation, when electrons are transferred by electron carriers from NADH or FADH2 to oxygen” What is Oxidative Phosphorylation? Oxidative phosphorylation is the final step in cellular respiration. It occurs in the mitochondria.
What is the overall function of oxidative phosphorylation?
· oxidative phosphorylation noun Definition of oxidative phosphorylation : the synthesis of ATP by phosphorylation of ADP for which energy is obtained by electron transport and which takes place in the mitochondria during aerobic respiration Examples of oxidative phosphorylation in a Sentence
What are the three products of oxidative phosphorylation?
· Oxidative Phosphorylation Definition. Oxidative Phosphorylation, also known as OXPHOS, refers to the redox reactions involving the flow of electrons along a series of membrane-bound proteins, coupled with the generation of Adenosine triphosphate (ATP). Oxidative phosphorylation is the fourth and final step in cellular respiration.
Why is oxidative phosphorylation called oxidative?
Potential energy In the mitochondrion, what the proton gradient does is facilitate the production of ATP from ADP and Pi. This process is known as oxidative phosphorylation, because the phosphorylation of ADP to ATP is dependent on the oxidative reactions occurring in the mitochondria.
What is oxidative phosphorylation in simple words?
Definition of oxidative phosphorylation : the synthesis of ATP by phosphorylation of ADP for which energy is obtained by electron transport and which takes place in the mitochondria during aerobic respiration.
What is oxidative transport?
Oxidative phosphorylation is made up of two closely connected components: the electron transport chain and chemiosmosis. In the electron transport chain, electrons are passed from one molecule to another, and energy released in these electron transfers is used to form an electrochemical gradient.
What is oxidative used for?
Oxidation reduces the biochemical oxygen demand (BOD) of wastewater, thus, the toxicity of some impurities. In this treatment, some impurities are converted to carbon dioxide, water, and bio-solids. Chemical oxidation is also frequently used for disinfection.
Why is the electron transport chain called oxidative phosphorylation?
Oxidative phosphorylation is a process involving a flow of electrons through the electron transport chain, a series of proteins and electron carriers within the mitochondrial membrane. This flow of electrons allows the electron transport chain to pump protons to one side of the mitochondrial membrane.
What is oxidative phosphorylation quizlet?
Oxidative Phosphorylation. The process by which ATP is formed as a result of the transfer of electrons from NADH and FADH₂ to O₂ by a series of electron carriers. Proton-motive force. transfer of electrons leads to the pumping of protons. Takes place across the inner mitochondrial membrane that's used to synthesize ATP.
How is ATP produced in the electron transport system and oxidative phosphorylation?
The process of forming ATP from the electron transport chain is known as oxidative phosphorylation. Electrons carried by NADH + H+ and FADH2 are transferred to oxygen via a series of electron carriers, and ATPs are formed. Three ATPs are formed from each NADH + H+, and two ATPs are formed for each FADH2 in eukaryotes.
What is oxidative phosphorylation and where does it occur?
Oxidative phosphorylation occurs in the mitochondria of all animal and plant tissues, and is a coupled process between the oxidation of substrates and production of ATP. As the Kreb's cycle runs, hydrogen ions (or electrons) are carried by the two carrier molecules NAD or FAD to the electron transport pumps.
What are oxidants in the body?
Oxidants are reactive molecules that are produced both inside your body and the environment that can react with other cellular molecules in your body such as protein, DNA and lipids. When it does that, it damages molecules and it's what causes disease and inflammation.
Is oxidative phosphorylation aerobic or anaerobic?
AerobicAerobic vs anaerobic respirationAerobicAnaerobicLocationCytoplasm (glycolysis) and mitochondriaCytoplasmStagesGlycolysis (anaerobic), Krebs cycle, oxidative phosphorylationGlycolysis, fermentationATP producedLarge amount (36 ATP)Small amount (2 ATP)2 more rows
What does phosphorylation mean simple?
(fos-FOR-ih-LAY-shun) A process in which a phosphate group is added to a molecule, such as a sugar or a protein.
What is oxidative phosphorylation and where does it occur?
Oxidative phosphorylation occurs in the mitochondria of all animal and plant tissues, and is a coupled process between the oxidation of substrates and production of ATP. As the Kreb's cycle runs, hydrogen ions (or electrons) are carried by the two carrier molecules NAD or FAD to the electron transport pumps.
What is oxidative phosphorylation Ncert?
Oxidative Phosphorylation Definition “Oxidative phosphorylation is the process of ATP formation, when electrons are transferred by electron carriers from NADH or FADH2 to oxygen”
How do you say oxidative phosphorylation?
Phonetic spelling of oxidative phosphorylationox-id-at-ive phos-phoryla-tion. Brock Sur.oxidative phosphorylation. Themba Mabena.ox-ida-tive phos-pho-ry-la-tion.
Where does oxidative phosphorylation occur?
Oxidative phosphorylation is the final step in cellular respiration. It occurs in the mitochondria. It is linked to a process known as electron transport chain. The electron transport system is located in the inner mitochondrial membrane.
Which biochemical process releases a huge amount of energy on oxidation?
Electron Transport Chain. Most of the biochemical catabolic processes like the citric acid cycle, glycolysis, beta-oxidation, etc. produce the coenzyme NADH. It consists of electrons having high transfer potential. These reactions release a huge amount of energy on oxidation.
What is the process of moving electrons from a higher energy level to a lower energy level?
Electron Transport and Proton Pumping. The electrons move from a higher energy level to a lower energy level, thereby releasing energy. Some of the energy is used to move the electrons from the matrix to the intermembrane space. Thus, an electrochemical gradient is established.
Where do reduced NADH and FADH2 transfer electrons?
Reduced NADH and FADH2 transfer their electrons to molecules near the beginning of the transport chain. After transferring the electrons, they get oxidised to NAD+ and FAD and are utilised in other steps of cellular respiration.
How is energy transferred from the electron transport chain to ATP synthase?
As a result, energy will be transmitted from the electron transport chain to ATP synthase by the movement of proteins. This process is termed as chemiosmosis. Endergonic Process is a chemical reaction in which energy is absorbed. There will be a change in free energy and it is always positive.
What is the purpose of oxidative phosphorylation?
Oxidative phosphorylation uses these molecules to produce ATP, which is used throughout the cell when ever energy is needed. During oxidative phosphorylation, electrons are transferred from the electron donors to a series of electron acceptors in a series of redox reactions ending in oxygen as the last acceptor.
How does oxidative phosphorylation work?
Oxidative phosphorylation works by using energy -releasing chemical reactions to drive energy-requiring reactions. The two sets of reactions are said to be coupled. This means one cannot occur without the other. The chain of redox reactions driving the flow of electrons through the electron transport chain, from electron donors such as NADH to electron acceptors such as oxygen and hydrogen (protons), is an exergonic process – it releases energy, whereas the synthesis of ATP is an endergonic process, which requires an input of energy. Both the electron transport chain and the ATP synthase are embedded in a membrane, and energy is transferred from the electron transport chain to the ATP synthase by movements of protons across this membrane, in a process called chemiosmosis. A current of protons is driven from the negative N-side of the membrane to the positive P-side through the proton-pumping enzymes of the electron transport chain. The movement of protons creates an electrochemical gradient across the membrane, which is often called the proton-motive force. It has two components: a difference in proton concentration (a H + gradient, Δ pH) and a difference in electric potential, with the N-side having a negative charge.
What is the final step in the oxidative phosphorylation pathway?
ATP synthase , also called complex V, is the final enzyme in the oxidative phosphorylation pathway. This enzyme is found in all forms of life and functions in the same way in both prokaryotes and eukaryotes. The enzyme uses the energy stored in a proton gradient across a membrane to drive the synthesis of ATP from ADP and phosphate (P i ). Estimates of the number of protons required to synthesize one ATP have ranged from three to four, with some suggesting cells can vary this ratio, to suit different conditions.
What is the difference between eukaryotic and prokaryotic oxidative phosphorylation?
The main difference between eukaryotic and prokaryotic oxidative phosphorylation is that bacteria and archaea use many different substances to donate or accept electrons. This allows prokaryotes to grow under a wide variety of environmental conditions. In E. coli, for example, oxidative phosphorylation can be driven by a large number of pairs of reducing agents and oxidizing agents, which are listed below. The midpoint potential of a chemical measures how much energy is released when it is oxidized or reduced, with reducing agents having negative potentials and oxidizing agents positive potentials.
How does phosphorylation change the equilibrium?
This phosphorylation reaction is an equilibrium, which can be shifted by altering the proton-motive force. In the absence of a proton-motive force, the ATP synthase reaction will run from right to left, hydrolyzing ATP and pumping protons out of the matrix across the membrane. However, when the proton-motive force is high, the reaction is forced to run in the opposite direction; it proceeds from left to right, allowing protons to flow down their concentration gradient and turning ADP into ATP. Indeed, in the closely related vacuolar type H+-ATPases, the hydrolysis reaction is used to acidify cellular compartments, by pumping protons and hydrolysing ATP.
How many ATPs are produced by oxidative phosphorylation?
Glycolysis produces only 2 ATP molecules, but somewhere between 30 and 36 ATPs are produced by the oxidative phosphorylation of the 10 NADH and 2 succinate molecules made by converting one molecule of glucose to carbon dioxide and water, while each cycle of beta oxidation of a fatty acid yields about 14 ATPs.
What is the phosphorylation of ADP to ATP?
The phosphorylation of ADP to ATP that accompanies the oxidation of a metabolite through the operation of the respiratory chain. Oxidation of compounds establishes a proton gradient across the membrane, providing the energy for ATP synthesis. The electron transport chain in the cell is the site of oxidative phosphorylation.
Examples of oxidative phosphorylation in a Sentence
Recent Examples on the Web Memory T cells, for instance, typically favor oxidative phosphorylation and consume fatty acids. — Mitch Leslie, Science | AAAS, 29 Mar. 2018 Metformin curtails oxidative phosphorylation, whereas 2DG squelches glycolysis. — Mitch Leslie, Science | AAAS, 29 Mar. 2018
First Known Use of oxidative phosphorylation
Subscribe to America's largest dictionary and get thousands more definitions and advanced search—ad free!
What is oxidative phosphorylation?
Oxidative Phosphorylation, also known as OXPHOS, refers to the redox reactions involving the flow of electrons along a series of membrane-bound proteins, coupled with the generation of Adenosine triphosphate (ATP).
What are the electron carriers of oxidative phosphorylation?
The most common electron carriers associated with oxidative phosphorylation are nicotinamide adenine dinucleotide (NAD +) and flavin adenine dinucleotide (FAD). When NAD + and FAD accept electrons, they become reducing agents (NADH and FADH 2) that are capable of transferring these electrons to molecules that have a high affinity for them. ...
What is the final resting spot for electrons that enter the system through NADH and FADH 2?
Water is the final resting spot for the electrons that entered the system through NADH and FADH 2 and is either used in the cell’s metabolic pathways or is excreted from the body. This is a schematic representation of the complexes involved in oxidative phosphorylation and ATP generation.
What is the energy released by oxidation?
Here, the chemical energy in the organic molecules is released gradually, through step-wise oxidation to carbon dioxide. The process also generates a number of high-energy electrons, which are harnessed by special molecules called electron carriers. The most common electron carriers associated with oxidative phosphorylation are nicotinamide adenine ...
What is the fourth step of cellular respiration?
Oxidative phosphorylation is the fourth and final step in cellular respiration. While respiration can be represented as the simple ‘combustion’ of carbohydrates to form carbon dioxide and water, the cell cannot afford to release all the chemical energy stored in carbohydrates in a single step, since it would irreversibly damage ...
Which protein is involved in the transfer of electrons from NADH to ubiquinone?
Between the two electron carriers, NADH has a lower reduction potential, and releases electrons to complex I. Complex I is also known as NADH:quinone oxidoreductase and is instrumental in the transfer the electrons from NADH to a protein called ubiquinone. This enormous membrane-bound complex consists of 46 polypeptide chains and can combine the acceptance of two electrons from NADH with the movement of 4 H + ions from the matrix to the inter-membrane space. Each of these four protons is pumped through a separate, dedicated channel.
How many phosphate groups are in ATP?
ATP has three phosphate groups in close physical proximity to one another. The addition of every phosphate group needs to be coupled with some other energy-releasing or exergonic reaction, since the natural repulsion between the negatively charged phosphate groups needs to be overcome.
Which process produces more ATP than any other part of cellular respiration?
The most vital part of this process is the electron transport chain, which produces more ATP than any other part of cellular respiration.
What is the process of releasing energy from electrons?
Oxidative phosphorylation is the metabolic pathway in which electrons are transferred from electron donors to electron acceptors in redox reactions; this series of reactions releases energy which is used to form ATP. There are four protein complexes (labeled complex I-IV) in the electron transport chain, which are involved in moving electrons ...
What is the final step of aerobic respiration?
The electron transport chain is the final component of aerobic respiration and is the only part of glucose metabolism that uses atmospheric oxygen. Electron transport is a series of redox reactions that resemble a relay race. Electrons are passed rapidly from one component to the next to the endpoint of the chain, where the electrons reduce molecular oxygen, producing water. This requirement for oxygen in the final stages of the chain can be seen in the overall equation for cellular respiration, which requires both glucose and oxygen.
Which compound accepts electrons from both complex I and complex II and delivers them to complex III?
Ubiquinone (Q) accepts the electrons from both complex I and complex II and delivers them to complex III.
Where does oxidative phosphorylation occur?
It occurs in mitochondria.
How many articles are there on uncoupling agents of oxidative phosphorylation?
Four articles on uncoupling agents of oxidative phosphorylationwere found.
What is the process of cellular respiration?
The process of cellular respiration occurring within the MITOCHONDRIAand responsible for the production of adenosine triphosphate (ATP). In this process energy derived from the oxidation of hydrogen, to form water, is transferred to ATP.
Which phosphorylation is sensitive to calcium and accumulation within mitochondria?
Oxidative phosphorylationis sensitive to calcium and accumulation within mitochondria is known to stimulate aerobic ATP production by the ETC [108, 109].
What is the energy released by the flow of electrons to O2and the dehydrogenation of various substrates
formation of high-energy phosphoric bonds (for example, in pyrophosphates) from the energy released by the flow of electrons to O2and the dehydrogenation (i.e., oxidation) of various substrates, most notably isocitric acid, α-ketoglutaric acid, succinic acid, and malic acid in the tricarboxylic acid cycle.
Which enzymes synthesize ATP from ADP and inorganic phosphate during the oxidation of
The process in cell metabolism by which respiratory enzymes in the mitochondria synthesize ATP from ADP and inorganic phosphate during the oxidation of NADH by molecular oxygen.
Where does ATP form?
The formation of ATP within mitochondria using energy derived from the electron transport chain
What is the process of oxidative phosphorylation?
Almost all aerobic organisms carry out oxidative phosphorylation. This pathway is probably so pervasive because it is a highly efficient way of releasing energy, compared to alternative fermentation processes such as anaerobic glycolysis. During oxidative phosphorylation, electrons are transferred from electron donors to electron acceptors such as oxygen, in redox reactions. These redox reactions release energy, which is used to form ATP. In eukaryotes, these redox reactions are carried out by a series of protein complexes within the cell's intermembrane wall mitochondria, whereas, in prokaryotes, these proteins are located in the cells' intermembrane space. These linked sets of proteins are called electron transport chains. In eukaryotes, five main protein complexes are involved, whereas in prokaryotes many different enzymes are present, using a variety of electron donors and acceptors.
What is the numerical value of oxidative phosphorylation in Pythagorean numerology?
The numerical value of oxidative phosphorylation in Pythagorean Numerology is: 5
What are the reducing equivalents of oxidative phosphorylation?
Most of the reducing equivalents used in oxidative phosphorylation come from intramitochondrial NADH , which is produced by the citric acid cycle, fatty acid oxidation, and amino acid metabolism. The intramitochondrial [NAD+]/[NADH] ratio is regulated and is typically maintained at a redox potential near −0.35 ± 0.03. All of the reactions within oxidative phosphorylation are near equilibrium except the reduction of molecular oxygen to the bound peroxide in cytochrome c oxidase. The redox components of the respiratory chain are organized in groups with half‐reduction potentials near −0.3 V, −0.00 V, 0.25 V, and 0.6 V, the most positive being the bound peroxide intermediate of the oxidase. Within each group of redox components the exchange rates sufficiently exceed the net flux through oxidative phosphorylation that they form an isopotential ‘pool’ of reducing equivalents. Feedback by the energy state is applied equally and independently to each step, resulting in 3 stage amplification of the signal.
What is the role of phosphorylation in the ATP pathway?
The pathway incorporates three consecutive near equilibrium steps for moving reducing equivalents between the intramitochondrial [NAD+]/[NADH] pool to molecular oxygen, with irreversible reduction of oxygen to bound peroxide at cytochrome c oxidase determining the net flux. Net flux (oxygen consumption rate) is determined by demand for ATP, with feedback by the energy state ([ATP]/[ADP][Pi]) regulating the pathway. This feedback affects the reversible steps equally and independently, resulting in the rate being coupled to ([ATP]/[ADP][Pi])3. With increasing energy state, oxygen consumption decreases rapidly until a threshold is reached, above which there is little further decrease. In most cells, [ATP] and [Pi] are much higher than [ADP] and change in [ADP] is primarily responsible for the change in energy state. As a result, the rate of ATP synthesis, plotted against [ADP], remains low until [ADP] reaches about 30 μmand then increases rapidly with further increase in [ADP]. The dependencies on energy state and [ADP] near the threshold can be fitted by the Hill equation with a Hill coefficients of about −2.6 and 4.2, respectively. The homeostatic set point for metabolism is determined by the threshold, which can be modulated by the PO2and intramitochondrial [NAD+]/[NADH]. The ability of oxidative phosphorylation to precisely set and maintain metabolic homeostasis is consistent with it being permissive of, and essential to, development of higher plants and animals.
What is the energy state of ATP?
In cells under physiological conditions, where the cytochrome c turnover is 5–10 s−1, the energy state is near 4 × 104 m−1 (a free energy of hydrolysis of ATP near −14.6 kcal mol−1or −61.1 kJ mol−1). For resting conditions, the potential difference across each of the three steps of oxidative phosphorylation is near 0.32 V. As a result, approximately 0.96 V of the available 1.165 V is used to synthesize ATP. This is a high, about 80%, overall coupling efficiency. The about 20% loss is as heat released when oxygen is reduced to the bound peroxide intermediate of cytochrome c oxidase (step 4). This loss results in the reaction being irreversible and therefore responsible for regulation of the metabolic flux. Because there are three sequential steps in the respiratory chain and they are all near equilibrium, feedback of the energy state is applied equally and independently to all three steps. A decrease in energy state that would cause a 3‐fold increase in the flux through one step results in an increase of 33or 27 applied to all 3 steps. This amplification of the feedback response greatly decreases the amount that the energy state needs to decrease in order to evoke particular increase in ATP synthesis.
What are the metabolic pathways?
Metabolic pathways typically involve many different reactions and metabolites. As emphasized by Imre Lakatos (1970, 1978), in biology the collection and interpretation of experimental data involves large numbers of assumptions/hypotheses, many of which are not stated or are presented as facts. Core hypotheses, those which are considered most important, strongly influence the interpretation given to sets of experimental data. Perceived inconsistencies between data and the core hypothesis result in addition of auxiliary assumptions/hypotheses designed to remove the inconsistency and protect the core hypothesis. These auxiliary assumptions/hypotheses constitute the protective belt of a Lakatosian Research Programme. Challenges to the research programme are mostly directed toward the auxiliary assumptions/hypotheses in the protective belt, and the research programme fails when the protective belt becomes seriously eroded.
How is the Lakatosian research programme relevant to the study of metabolism?
Relevance of the Lakatosian Research Programme to the study of metabolism is readily observed in the literature . Metabolic pathways involve a large number of variables and investigators are limited by not being able to measure all of the variables, limited accuracy of the measured values, variations among cell types and other sources of uncertainty, each of which requires additional hypotheses/assumptions. Many of these limitations can be overcome by building a computational model. Properly designed computational models involve: (1) listing all of the metabolites and metabolic transitions considered to be responsible for the regulating flux and; (2) writing equations that explicitly quantify the contributions of each to the whole. This process makes clear the hypotheses and assumptions made. In addition, it forces proposed interpretations of the data to recognize and account for all of the relevant parameters, including those not measured due to limitations in technology and/or experimental design. Computational models should be developed using Occam's razor because each variable more than the required minimum increases both the uncertainty for any given solution and the number of possible solutions when fitting the model to a data set. Occam's razor requires: (1) determining the minimum number of chemically and mechanistically sound equations required to quantify the behaviour; (2) identification of the minimum number of variables with important regulatory roles; and (3) confirmation that the resulting computational model predicts behaviour consistent with experimental observation. Once a minimal ‘core’ model has been developed and the values for the essential parameters determined, additional complexities can be ‘grafted’ onto the minimal model. Because the parameters of the minimal model have already been determined, only the ‘add on’ complexity needs to be quantified and evaluated and this can be readily done. Successful models are ones that predict relationships among the metabolites that are chemically reasonable, quantitative, and consistent with existing data. This consistency provides empirical support for the hypotheses/assumptions made in developing the model. It also provides justification for using the model to conduct virtual experiments prior to doing the actual experiments. Such virtual experiments predict outcomes, an invaluable resource for optimizing experimental design and interpreting the resulting data.
Does the mitochondrial model contain ad hoc parameters?
It does not contain any ad hocparameters that can be varied in order to ‘fit’ the model to different sets of data. The parameter values obtained from isolated mitochondria are used for all different cell and tissue types with the exception of the metabolite and cytochrome concentrations, which are cell and tissue specific. The internal parameters in the model were obtained by fit to experimental data for oxidative phosphorylation as a whole, but with the values constrained to be consistent with available model independent measurements.
Does oxidative phosphorylation predict respiratory rates?
It has been shown to predict behaviour of oxidative phosphorylation that is consistent with the measured energy state and respiratory rates in many tissues as well as with changes that occur during the work‐to‐rest (Wilson, 2016) and rest‐to‐work (Wilson, 2015a) transitions in oxidative skeletal muscle.

Overview
Oxidative phosphorylation or electron transport-linked phosphorylation or terminal oxidation is the metabolic pathway in which cells use enzymes to oxidize nutrients, thereby releasing chemical energy in order to produce adenosine triphosphate (ATP). In eukaryotes, this takes place inside mitochondria. Almost all aerobic organismscarry out oxidative phosphorylation. This pathway is so p…
Chemiosmosis
Oxidative phosphorylation works by using energy-releasing chemical reactions to drive energy-requiring reactions. The two sets of reactions are said to be coupled. This means one cannot occur without the other. The chain of redox reactions driving the flow of electrons through the electron transport chain, from electron donors such as NADH to electron acceptors such as oxygen and hydrogen (protons), is an exergonicprocess – it releases energy, whereas the synthesis of A…
Electron and proton transfer molecules
The electron transport chain carries both protons and electrons, passing electrons from donors to acceptors, and transporting protons across a membrane. These processes use both soluble and protein-bound transfer molecules. In mitochondria, electrons are transferred within the intermembrane space by the water-soluble electron transfer protein cytochrome c. This carries …
Eukaryotic electron transport chains
Many catabolic biochemical processes, such as glycolysis, the citric acid cycle, and beta oxidation, produce the reduced coenzyme NADH. This coenzyme contains electrons that have a high transfer potential; in other words, they will release a large amount of energy upon oxidation. However, the cell does not release this energy all at once, as this would be an uncontrollable reaction. Ins…
Prokaryotic electron transport chains
In contrast to the general similarity in structure and function of the electron transport chains in eukaryotes, bacteria and archaea possess a large variety of electron-transfer enzymes. These use an equally wide set of chemicals as substrates. In common with eukaryotes, prokaryotic electron transport uses the energy released from the oxidation of a substrate to pump ions across a membrane and generate an electrochemical gradient. In the bacteria, oxidative phosphorylation in Escherichia …
ATP synthase (complex V)
ATP synthase, also called complex V, is the final enzyme in the oxidative phosphorylation pathway. This enzyme is found in all forms of life and functions in the same way in both prokaryotes and eukaryotes. The enzyme uses the energy stored in a proton gradient across a membrane to drive the synthesis of ATP from ADP and phosphate(Pi). Estimates of the number of protons required to synthesize one ATP have ranged from three to four, with some suggesting c…
Oxidative phosphorylation - energetics
The energy released in oxidative phosphorylation can mostly be attributed to O2 with its relatively weak double bond. The transport of electrons from redox pair NAD / NADH to the final redox pair 1/2 O2/ H2O can be summarized as
1/2 O2 + NADH + H → H2O + NAD
The potential difference between these two redox pairs is 1.14 volt, which is equivalent to -52 kc…
Reactive oxygen species
Molecular oxygen is an ideal terminal electron acceptor because it is a strong oxidizing agent. The reduction of oxygen does involve potentially harmful intermediates. Although the transfer of four electrons and four protons reduces oxygen to water, which is harmless, transfer of one or two electrons produces superoxide or peroxide anions, which are dangerously reactive.
(7)
Molecular oxygen is an ideal terminal electron acceptor because it is a strong oxidizing agent. The reduction of oxygen does involve potentially harmful intermediates. Although the transfer of four electrons and four protons reduces oxygen to water, which is harmless, transfer of one or two electrons produces superoxide or peroxide anions, which are dangerously reactive.
(7)