An Overview of the Blood Clotting Process
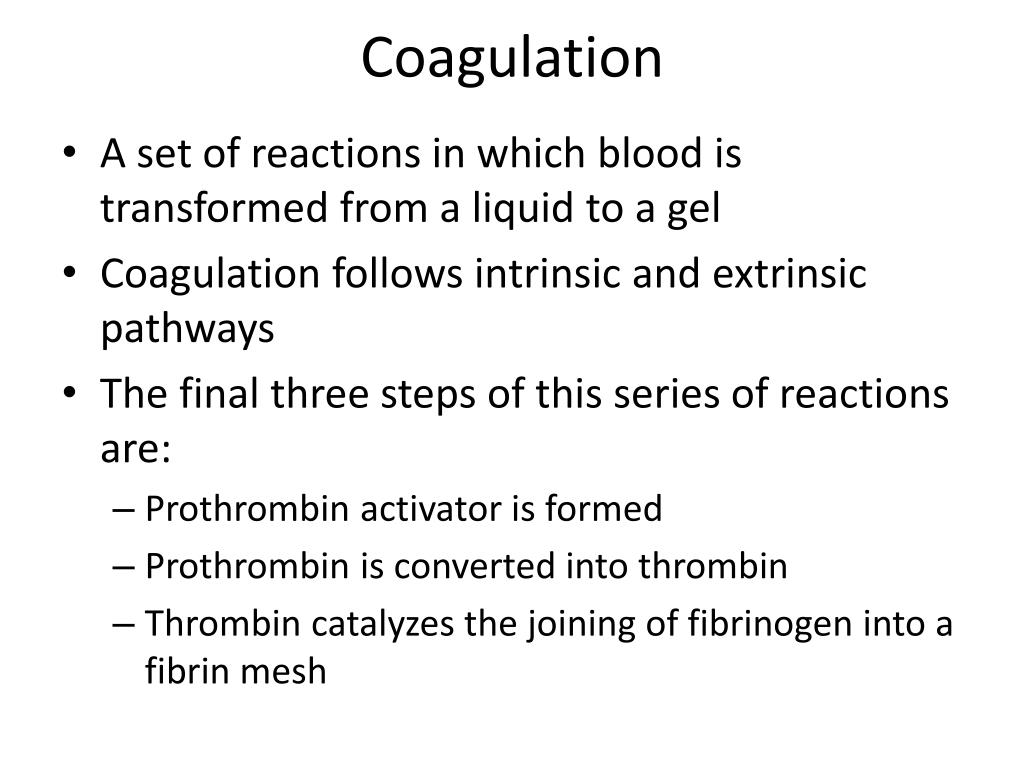
- A complex known as a prothrombin activator is produced by a long sequence of chemical reactions.
- The prothrombin activator converts a blood protein called prothrombin into another protein called thrombin.
- Thrombin converts a soluble blood protein called fibrinogen into an insoluble protein called fibrin.
What is the function of prothrombin?
Prothrombin. Prothrombin, glycoprotein (carbohydrate-protein compound) occurring in blood plasma and an essential component of the blood-clotting mechanism. Prothrombin is transformed into thrombin by a clotting factor known as factor X or prothrombinase; thrombin then acts to transform fibrinogen, also present in plasma, into fibrin, which,...
How is prothrombin activator released from the body?
Prothrombin activator is released in the body by a cascade of chemical reactions in response to damage in a blood vessel. Guyton, Arthur; Hall, John (2006). Textbook of Medical Physiology (11th edition). p. 459.
What are the different types of prothrombin activators?
However, there are distinct groups of prothrombin activators classified by their requirement for cofactors [245]. Group A activators are cofactor-independent metalloproteinases which cleave at the Arg320 site to form meizothrombin, an active prothrombin–thrombin intermediate which converts autocatalytically to thrombin.
What is thrombomodulin and thrombin activation?
Thrombomodulin-dependent activation of protein C and thrombin-activatable fibrinolysis inhibitor (TAFI), platelet aggregation, antithrombin-III inhibition. . Prothrombin activation on the activated platelet surface optimizes expression of procoagulant activity. .

What does prothrombin activator convert?
Prothrombin is transformed into thrombin by a clotting factor known as factor X or prothrombinase; thrombin then acts to transform fibrinogen, also present in plasma, into fibrin, which, in combination with platelets from the blood, forms a clot (a process called coagulation).
What triggers the formation of prothrombin activator?
The intrinsic mechanism of prothrombin activator formation begins with trauma to the blood or exposure of blood to collagen in a traumatized vessel wall. This usually also results in damage to fragile platelets. The formation of a clot by this mechanism usually takes 1 to 6 minutes.
How is prothrombin activator formed quizlet?
1) Formation of prothrombinase (prothrombin activator). Initiated by either the extrinsic or the intrinsic pathway or both. (2) Conversion of prothrombin (a plasma protein formed by the liver) into the enzyme thrombin by prothrombinase.
How is prothrombin activated a level biology?
Clotting begins with platelets forming a plug and initiating the release of clotting factors such as thromboplastin. This is a plasma protein which turns prothrombin to its active state, thrombin.
What components make up the prothrombin activator complex?
The prothrombinase complex consists of the serine protease, Factor Xa, and the protein cofactor, Factor Va. The complex assembles on negatively charged phospholipid membranes in the presence of calcium ions.
Which vitamin is necessary for prothrombin formation?
Vitamin K helps to make various proteins that are needed for blood clotting and the building of bones. Prothrombin is a vitamin K-dependent protein directly involved with blood clotting.
How is tissue factor activated?
After vessel injury or tissue trauma the TF-FVIIa complex is the spark that triggers blood coagulation by activating both FX and FIX. FVII bound to TF is rapidly activated by a variety of coagulation proteases, although autoactivation by trace amounts of FVIIa may be the primary pathway.
What is the process of transforming thrombin into thrombin?
Prothrombin is transformed into thrombin by a clotting factor known as factor X or prothrombinase; thrombin then acts to transform fibrinogen, also present in plasma, into fibrin, which, in combination with platelets from the blood, forms a clot (a process called coagulation ).
What is the cause of a lack of prothrombin?
Hypoprothrombinemia, a deficiency in prothrombin, is characterized by a tendency to prolonged bleeding. It is usually associated with a lack of vitamin K, which is necessary for the synthesis of prothrombin in the liver cells. In adults the condition occurs most commonly in cases of obstructive jaundice, in which the flow of bile to the bowel is interrupted—bile being necessary for the intestinal absorption of vitamin K. It can also result from a general impairment in liver and intestinal-cell function or overdose of warfarinand related therapeutic anticoagulants.
What is the deficiency of prothrombin?
Hypoprothrombinemia, a deficiency in prothrombin, is characterized by a tendency to prolonged bleeding. It is usually associated with a lack of vitamin K, which is necessary for the synthesis of prothrombin in the liver cells.
When does prothrombin change to thrombin?
Under normal circumstances, prothrombin is changed into thrombin only when injury occurs to the tissues or circulatory system or both; therefore, fibrin and blood clots are not formed except in response to bleeding. Hypoprothrombinemia, a deficiency in prothrombin, is characterized by a tendency to prolonged bleeding.
What is an encyclopedia editor?
Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. ...
What is the mTAT rate of WB?
mIIa generation was also assessed using the mTAT ELISA ( Figure 6 B). WB generated mTAT at a rate of 1.2 ± 0.8nM/min and reached a maximum level of 14.7 ± 11.3nM. PPP reconstituted to a 40% hematocrit showed significant mTAT formation ( Table 4) compared with the corresponding WB, with a maximum rate of approximately 0.7 ± 0.2nM/min (approximately 58% of WB) and reaching a level of 9.1 ± 2.9nM (approximately 62% of WB). PPP alone showed a lesser amount of mTAT generation (maximum rate, 0.4 ± 0.2nM/min; maximum level, 4.4 ± 2.5nM, 27% of WB).
How is mIIa produced?
mIIa production by prothrombinase in purified systems has been studied extensively on synthetic phospholipid vesicles. 3, 15, 16 In the absence of AT, prothrombin is rapidly activated through the mIIa pathway, with maximum mIIa levels transiently reaching 40% of the final αIIa concentration. In Tf-activated WB, the generation of mIIa results in partitioning of the mIIa between the reaction with the stoichiometric inhibitor AT and rebinding to prothrombinase and conversion to αIIa, with subsequent inhibition resulting in αTAT. Figure 3 A shows the results of computational analysis quantifying the extent of this partitioning. Predicted time courses for αTAT and mTAT generation after a hypothetical 5pM Tf stimulus are shown. The computational analysis predicted that, although all of the prothrombin was converted through mIIa, a maximum of 11% of the mIIa was trapped as mTAT rather than being converted to αIIa and eventually trapped as αTAT. Differences in proteome composition also affect the relative distribution of mTAT and αTAT species. 17
What is the pathway of prothrombinase?
mIIa arises from initial cleavage at Arg320, yielding a membrane-binding enzyme; initial cleavage at Arg271 yields an inactive noncovalent complex of fragment 1.2 and prethrombin-2. Cleavage at the alternate Arg residue yields αIIa. 3 On synthetic phospholipid vesicles, prothrombin activation by IIase proceeds exclusively through the mIIa pathway, 3 with the probable mechanism involving mIIa dissociating from the IIase complex and then rebinding to IIase to yield αIIa. 4, 5 On washed platelets, prothrombin activation proceeds through the prethrombin-2 pathway, 6 with no detectable mIIa being released from the platelet surface. We confirmed these previous results with washed platelets under static and flow conditions. 7 However, mIIa has been observed in clotting blood, 8 suggesting that prothrombin activation in blood involves both cleavage pathways. The relative prevalence of these 2 pathways in blood has potentially interesting regulatory consequences for hemostasis. Although the anticoagulant potential of mIIa as a protein C activator when in complex with thrombomodulin is similar to that of αIIa, 5 mIIa displays poor activity toward the procoagulant substrates fibrinogen, platelets, and fV; displays a reduced vulnerability to antithrombin (AT); and is a more potent vasoconstrictor. 9
What intermediates are involved in prothrombin activation?
Prothrombin activation can proceed through the intermediates meizothrombin or prethrombin-2. To assess the contributions that these 2 intermediates make to prothrombin activation in tissue factor (Tf)–activated blood, immunoassays were developed that measure the meizothrombin antithrombin (mTAT) and α-thrombin antithrombin (αTAT) complexes. We determined that Tf-activated blood produced both αTAT and mTAT. The presence of mTAT suggested that nonplatelet surfaces were contributing to approximately 35% of prothrombin activation. Corn trypsin inhibitor–treated blood was fractionated to yield red blood cells (RBCs), platelet-rich plasma (PRP), platelet-poor plasma (PPP), and buffy coat. Compared with blood, PRP reconstituted with PPP to a physiologic platelet concentration showed a 2-fold prolongation in the initiation phase and a marked decrease in the rate and extent of αTAT formation. Only the addition of RBCs to PRP was capable of normalizing αTAT generation. FACS on glycophorin A–positive cells showed that approximately 0.6% of the RBC population expresses phosphatidylserine and binds prothrombinase (FITC Xa·factor Va). These data indicate that RBCs participate in thrombin generation in Tf-activated blood, producing a membrane that supports prothrombin activation through the meizothrombin pathway.
How to wash RBCs?
Washed RBCs were diluted to 2% in wash buffer and mixed with an equal volume of wash buffer containing 4, 8, 12, or 16μM phorbol 12-myristate 13-acetate (PMA; 2, 4, 6, 8μM final concentrations) and incubated at room temperature for 30 minutes. The PMA-treated cells were then centrifuged at 1100 g for 5 minutes before being washed 3 times. Treated cells were diluted to 2 × 10 6 cell/mL in RBC wash buffer and phosphatidylserine (PS) exposure was detected with FITC-bovine lactadherin or FITC-EGRck-fXa (15nM) in the presence of saturating FVa (20nM) on a flow cytometer (FC500; Beckman Coulter).
What was the procedure used for the WB activation experiments?
The procedure used for the WB activation experiments was a modification of that described by Brummel et al. 11 CTI blood or blood fractions were aliquoted into rocking tubes at 37°C containing 5pM relipidated Tf 1-243. Tubes were quenched 1:1 with 50mM EDTA, 20mM benzamidine, and 100μM Phe-Pro-Arg chloromethylketone at defined intervals for 20 minutes. Samples were centrifuged at 1200 g for 30 minutes at 4°C, and both the soluble and sedimented materials were frozen at −80°C.
Do platelets release MIIA?
Platelets are thought to provide the primary surface for prothrombin activation. The observation that the IIase activation pathway on platelets does not release mIIa raises 2 important questions: (1) what is the source of mIIa reported in clotting blood 7 and (2) how significant are nonplatelet cell surfaces to overall thrombin generation?
