Does silver react with potassium nitrate?
When an aqueous solution of potassium chloride (KCl) when mixed with silver nitrate (AgNO3) solution an insoluble white substance formed is silver chloride (AgCl). The chemical reaction are as follows : KCl (aq) + AgNO3 (aq) → KNO3 (aq) + AgCl (s).
Is silver nitrate an acid or base or salt?
Silver nitrate. AgNO3. Synonyms: Nitric acid silver(I) salt. CAS 7761-88-8. Molecular Weight 169.87. Browse Silver nitrate and related products at Merck.
Does salt react with silver nitrate?
The process involves an oxidation–reduction couple whereby a solution of an iron salt reduces aqueous silver nitrate to finely divided metallic silver. Click to see full answer. Regarding this, what is physical developer?
What is made when silver nitrate react with sodium chloride?
When silver nitrate and sodium chloride dissolve in water, the silver cations separate from the nitrate anions, and the sodium cations separate from the chloride anions 2. This occurs with all ionic compounds when they dissolve in water, and is called dissociation.

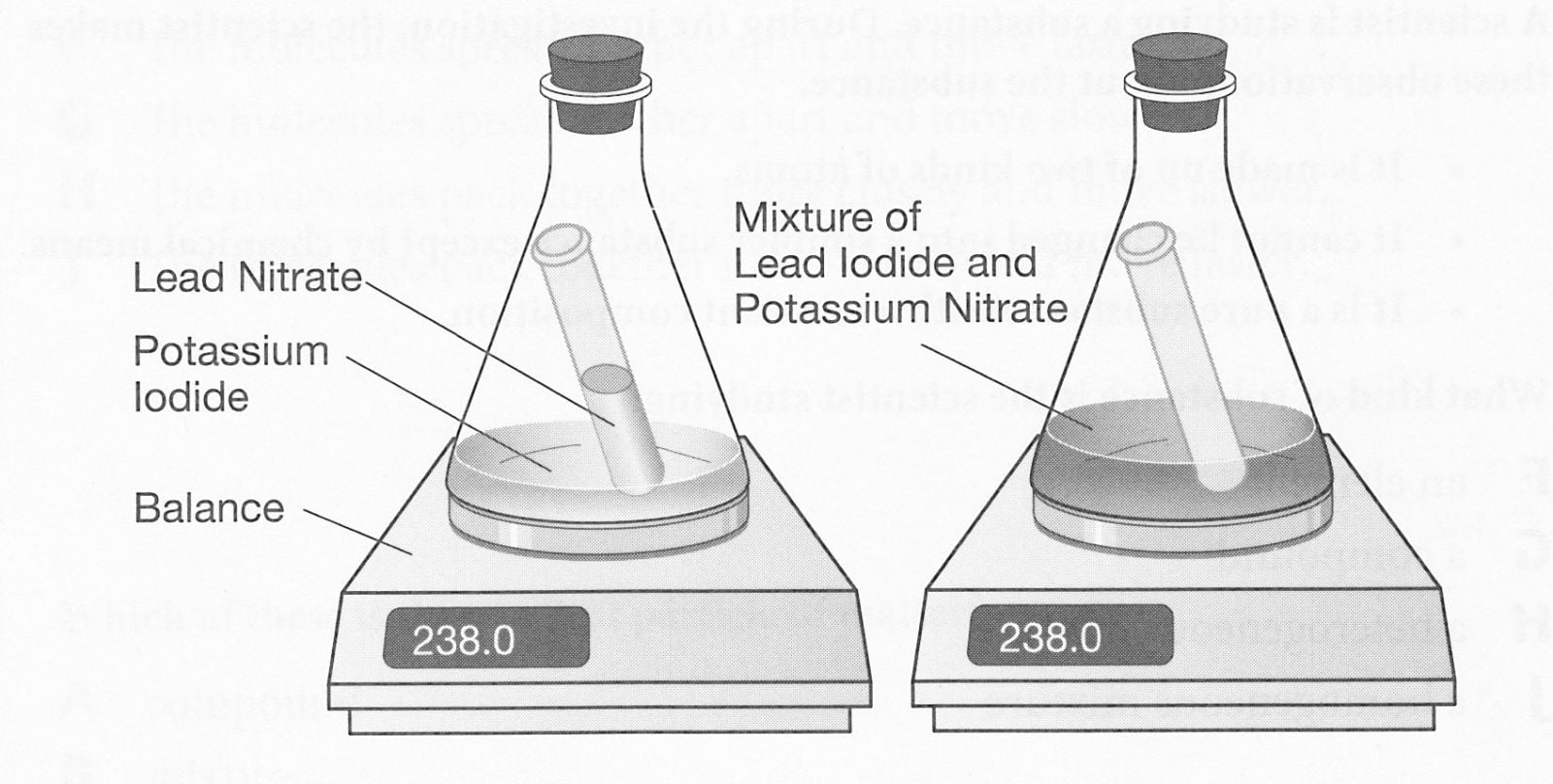
What happens when you mix silver nitrate and potassium iodide?
Aqueous silver nitrate reacts with aqueous potassium iodide in a double-replacement reaction to produce a precipitate of silver iodide.
Does potassium iodide and silver nitrate form A precipitate?
Aqueous solutions of potassium iodide and silver nitrate are mixed, forming the precipitate silver iodide.
What does AgNO3 and KI make?
AgI + KNO3How to Balance: AgNO3 + KI = AgI + KNO3 | Breslyn.org.
What happens when you mix silver nitrate and potassium nitrate?
The correct answer to this question is that when you mix silver nitrate and potassium chromate, precipitates of silver chromate appear.
What kind of reaction is precipitation?
A precipitation reaction is one in which dissolved substances react to form one (or more) solid products. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and are sometimes referred to as double displacement, double replacement, or metathesis reactions.
What is the Colour of silver iodide precipitate?
yellowishSilver iodide is prepared by reaction of an iodide solution (e.g., potassium iodide) with a solution of silver ions (e.g., silver nitrate). A yellowish solid quickly precipitates.
When AgNO3 solution is added to KI solution then the sol produced is?
When KI is added to AgNo3, a positive Sol of AgI will form (Ag+ will be preferred). This happens due to adsorption of Ag+ ions from the dispersion medium on the precipitate of silver iodide.
Does silver nitrate and sodium carbonate form a precipitate?
Here, sodium carbonate (Na2CO3) is added to silver nitrate (AgNO3). The result is a white precipitate.
What is a precipitate in chemistry?
Precipitate: In chemistry, a solid formed by a change in a solution, often due to a chemical reaction or change in temperature that decreases solubility of a solid. In meteorology a precipitate is liquid or solid water (rain, snow, etc.) falling from the sky. Fehling's solution is blue due to dissolved Cu2+.
Is silver nitrate explosive?
CONTAINERS MAY EXPLODE IN FIRE. Use water spray to keep fire-exposed containers cool. Silver Nitrate may ignite combustibles (wood, paper and oil). Silver Nitrate reacts with ACETYLENE, in the presence of AMMONIA, to form Silver Acetylide, a sensitive and powerful detonator.
Does silver nitrate and potassium chloride form a precipitate?
When we mix silver nitrate and potassium chloride together, a white precipitate of silver chloride forms. Calculate the mass of potassium chloride when two grams of silver chloride is precipitated.
What type of reaction is silver nitrate and potassium chloride?
double displacement reactionWhen an aqueous solution of potassium chloride KCl, when mixed with silver nitrate AgNO3 solution an insoluble AgCl formed as a white substance. This is an example of a double displacement reaction.
What precipitate forms when silver nitrate and potassium chromate solutions are mixed?
A precipitation reaction is a reaction that yields an insoluble product—a precipitate—when two solutions are mixed. When a colorless solution of silver nitrate is mixed with a yellow-orange solution of potassium dichromate, a reddish precipitate of silver dichromate is produced.
Is AgI soluble or insoluble?
insolubleAgI is practically insoluble in water. Only 3 x 10 -7 g will dissolve in 100mL of water at 20 °C. The solubility of most ionic compounds will increase as the temperature of the solvent increases.
What type of reaction occurs when potassium iodide reacts with silver nitrate to produce elemental iodine sliver metal and potassium nitrate?
1 Answer. Al E. They used to call this type of reaction a double replacement reaction.
What type of reaction is silver nitrate and potassium chloride?
double displacement reactionWhen an aqueous solution of potassium chloride KCl, when mixed with silver nitrate AgNO3 solution an insoluble AgCl formed as a white substance. This is an example of a double displacement reaction.
What happens when you mix potassium and silver?
exception to this rule is silver chloride. So when you mix silver nitrate and potassium chloride, you form a precipitate of silver chloride. The nitrate and the potassium do not react. How to entirely empty your bowels every morning (revealed). World renowned cardiologist explains how with at home trick.
What is the effect of AgNO3 on glass plates?
Later the same effect was obtained using AgNO3 in a gelatin smeared onto glass plates to obtain a “negative” image with dark regions of Ag2O forming where the bright light was and no Ag2O forming where the dark regions were.
What is the melting point of KNO3?
Silver nitrate has a melting point of 209.7°C, whereas potassium nitrate has a melting point of 334°C. And since KNO3 does not decompose below 550°C, you can heat the mix carefully to 220°C - 250°C. The AgNO3 will melt and flow away from the KNO3.
What type of reaction is ammonium made of?
They are each made of a cation and an anion. The type of reaction they may undergo is known as a double displacement reaction, in which the cations and anions can swap. So the ammonium could join with nitrate, and silver could join with chloride. The word equation would be:-.
Can nitrates be dissolved in water?
No. All nitrates are soluble in aqueous solution. If you combine potassium nitrate (KNO3) and barium nitrate (Ba (NO3)2), the ions will dissociate in the solution and will not react.
Do nitrates react with potassium?
The nitrate and the potassium do not react.
Is silver chloride soluble in potassium?
Chlorides are soluble. The famous exception to this rule is silver chloride. So when you mix silver nitrate and potassium chloride, you form a precipitate of silver chloride. The nitrate and the potassium do not react.
