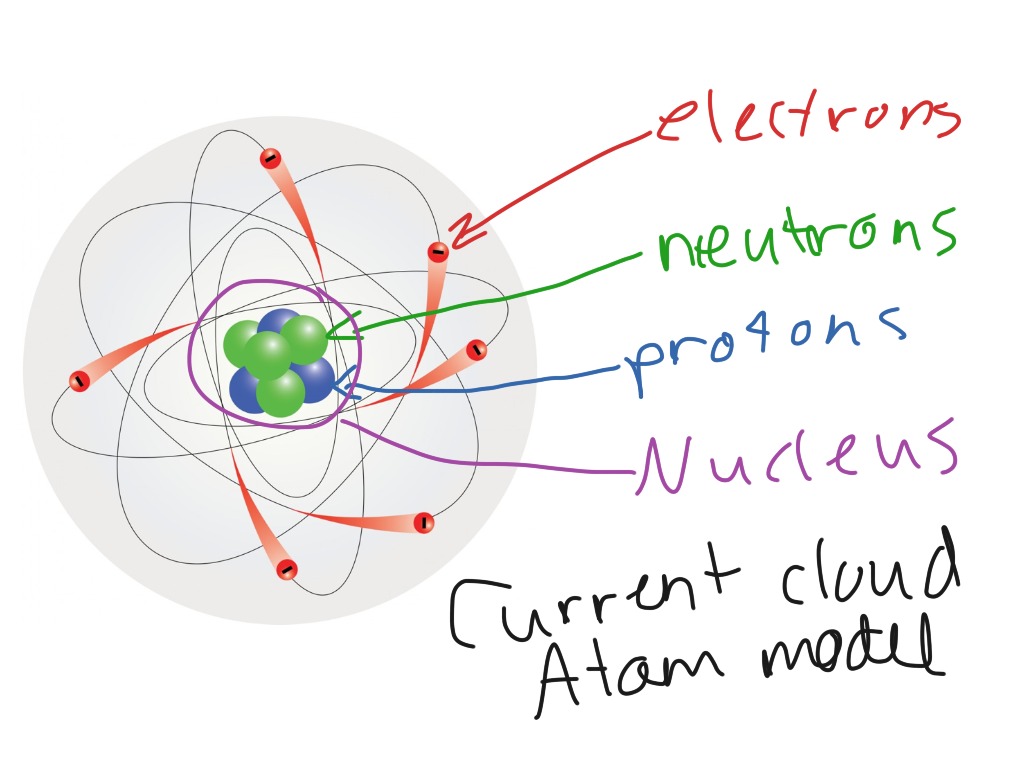
What does an electron cloud really look like?
What does an electron cloud look like? The modern model is also commonly called the electron cloud model. That’s because each orbital around the nucleus of the atom resembles a fuzzy cloud around the nucleus, like the ones shown in the Figure below for a helium atom.
What is the definition of electron cloud model?
Electron Cloud. Electron cloud model is a model of an atom, in which the atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.The electron cloud model says that we cannot know exactly where an electron is at any given time, but the electrons are more likely to be in specific areas.
Who propsed the electron cloud model?
Thanks to ongoing studies on the behavior of electrons, scientists began to propose theories whereby these elementary particles behaved in ways that defied classical, Newtonian physics. One such example is the Electron Cloud Model proposed by Erwin Schrodinger.
Why is the current atomic model call the electron cloud model?
The modern model is also commonly called the electron cloud model. That's because each orbital around the nucleus of the atom resembles a fuzzy cloud around the nucleus, like the ones shown in the Figure below for a helium atom. The densest area of the cloud is where the electrons have the greatest chances of being
See more
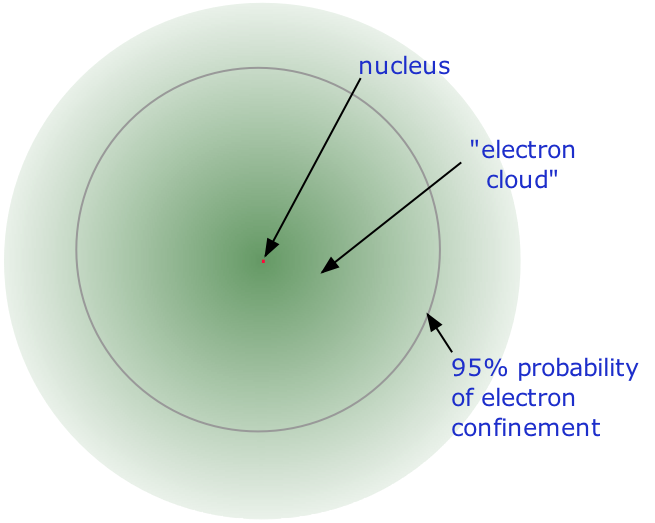
Why is it called an electron cloud?
An electron cloud kind of looks like a cloud. It is thicker in the center and fades out at the edges. The term cloud also describes the many possib...
Who came up with the electron cloud model?
Erwin Schrodinger came up with the electron cloud model, building on the work of several other physicists. Louis de Broglie's particle-wave duality...
What best describes an electron cloud?
The electron cloud is a particular area in which an electron is likely to be. We can't say exactly where an electron is, but we can use its wave fu...
What is another name for an electron cloud?
An electron cloud can be thought of as a probability field, the area in space where an electron is likely to be. In an atom, these fields, specific...
Why is it called the electron cloud model?
The electron cloud model shows a particular area in which an electron is likely to be. In a simple atom like Helium for instance, the probability f...
What is the electron cloud model used for?
The electron cloud is used to describe the behavior of electrons, and it is useful in building a model of the atom. The electron cloud shows the ar...
How does the electron cloud model differ from the Bohr model?
Based on quantum theory, which states that all matter has properties associated with a wave function, the Electron Cloud Model differs from the Bohr Model in that it does not define the exact path of an electron. Instead, it predicts the likely position of the location of the electron based on a function of probabilities.
Who was the first physicist to study electron cloud?
The Electron Cloud Model: During the 1920s, Austrian physicist Erwin Schrodinger became fascinated by the theories Max Planck, Albert Einstein, Niels Bohr, Arnold Sommerfeld, and other physicists. During this time, he also became involved in the fields of atomic theory and spectra, researching at the University of Zurich and then ...
Why is the plum pudding model named after the plum pudding?
And from this, the Plum Pudding Model was born, so named because it closely resembled the English desert that consists of plum cake and raisins.
Which model of matter disproved the notion that hydrogen atoms were the smallest unit of matter?
To explain the overall charge of the atom, which consisted of both positive and negative charges, Thompson proposed a model whereby the negatively charged “corpuscles” were distributed in a uniform sea of positive charge – known as the Plum Pudding Model.
Which model of the atom is surrounded by an equal number of electrons in orbital shells?
the Bohr Model ).
What was the smallest known atom?
However, most scientists ventured that this unit would be the size of the smallest known atom – hydrogen .
Where are electrons likely to be found?
Hence, their locations could only be described as being part of a ‘cloud’ around the nucleus where the electrons are likely to be found.
What is an electron cloud model?
The model is used to describe the probable locations of electrons around the atomic nucleus. The electron cloud is also defined as the region where an electron forms a three-dimensional standing wave, the one that does not move relative to the atomic nucleus. The model does not depict electrons as particles moving around the nucleus in a fixed orbit. Based on quantum mechanics, it gives the probable location of electrons represented by an ‘electron cloud’.
What does the cloud of electrons mean?
It consisted of a nucleus surrounded by clouds of electrons. The clouds indicate the probable positions of electrons in an atom. Greater density of electrons in a certain area is indicative of a higher probability of finding electrons in that atomic region. ► Werner Heisenberg, best known for his uncertainty principle, ...
What did Rutherford discover about atoms?
Till that time, atoms were believed to be the indivisible units of matter. His revolutionary discovery proved the conventional theory wrong. ► In 1909, Rutherford brought out the fact that the positive charge and the mass of an atom is concentrated towards its center, and that electrons orbit around the atomic center.
What is an orbital in an atom?
An orbital is a mathematical function that describes the wave-like behavior of electrons in an atom.
What is the force of attraction between the electrons and the nuclear protons?
It’s an electromagnetic force; a force of attraction that exists between the electrons and the nuclear protons, and binds them to the atom. The attractive force of an electron is directly proportional to its distance from the atomic nucleus. Hence the energy required to separate an electron from the atom varies inversely with its distance from ...
What binds the subatomic particles in an atom together?
It’s an electromagnetic force ; a force of attraction that exists between the electrons and the nuclear protons, and binds them to the atom. The attractive force of an electron is directly proportional to its distance from the atomic nucleus. Hence the energy required to separate an electron from the atom varies inversely with its distance from the nucleus.
What is an electron cloud?
An electron cloud is the region of negative charge surrounding an atomic nucleus that is associated with an atomic orbital. It is defined mathematically, describing a region with a high probability of containing electrons .
Why do chemists use electron cloud models?
Chemists use the electron cloud model to map out the atomic orbitals for electrons; these probability maps are not all spherical. Their shapes help predict the trends seen in the periodic table. Cite this Article. Format.
What is the cloud of electrons?
The electron cloud is the location around the nucleus that contains negatively-charged electrons.
When was the electron cloud invented?
It is defined mathematically, describing a region with a high probability of containing electrons . The phrase "electron cloud" first came into use around 1925, when Erwin Schrödinger and Werner Heisenberg were seeking for a way to describe the uncertainty of the position of electrons in an atom.
Where are electrons found in the cloud?
In the cloud model, there are regions where an electron may likely be found, but it's theoretically possible for it to be located anywhere, including inside the nucleus .