
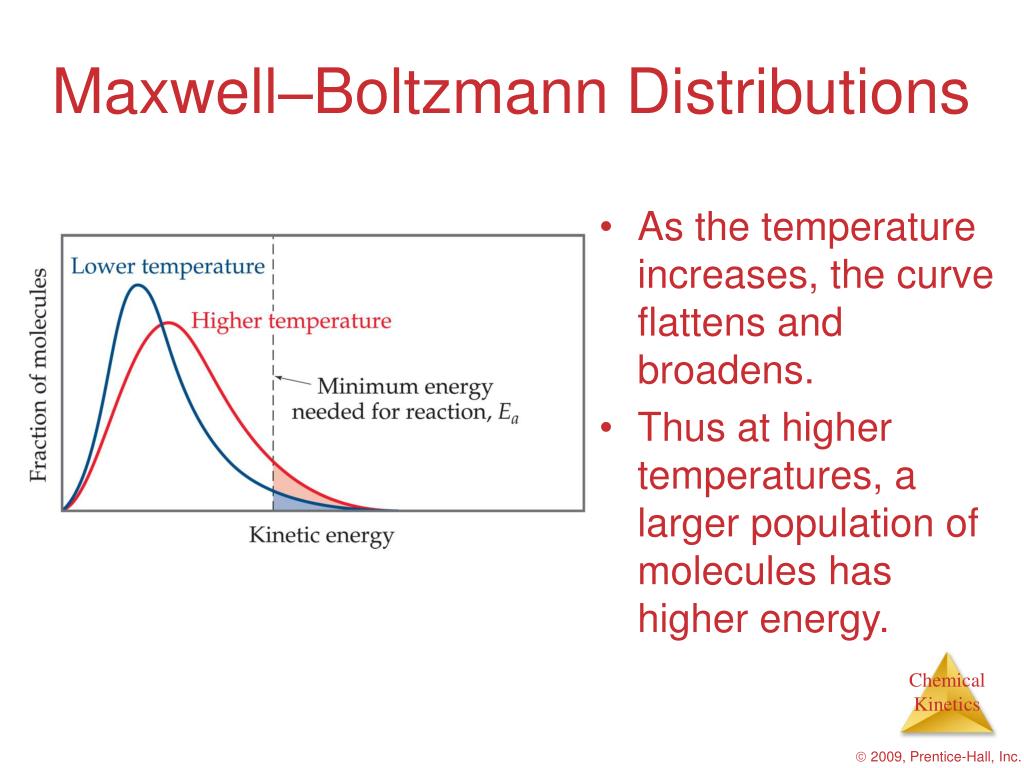
The Maxwell-Boltzmann distribution
Boltzmann distribution
In statistical mechanics and mathematics, a Boltzmann distribution (also called Gibbs distribution) is a probability distribution, probability measure, or frequency distribution of particles in a system over various possible states.
What is a Maxwell-Boltzmann distribution?
A Maxwell-Boltzmann Distribution is a probability distribution used for describing the speeds of various particles within a stationary container at a specific temperature.
Why is the average speed on the Maxwell-Boltzmann graph right?
The reason the average speed is located to the right of the peak is due to the longer "tail" on the right side of the Maxwell-Boltzmann distribution graph. This longer tail pulls the average speed slightly to the right of the peak of the graph.
What does the Y-axis of the Maxwell-Boltzmann graph mean?
The y-axis of the Maxwell-Boltzmann graph can be thought of as giving the number of molecules per unit speed. So, if the graph is higher in a given region, it means that there are more gas molecules moving with those speeds. [Wait, isn't the probability equal to zero for a gas molecule to be moving at any exact speed?]
What is Maxwell distribution of velocities?
Maxwell distribution of velocities states that the gaseous molecules inside the system travel at different velocities.
What does the Maxwell-Boltzmann distribution show energy?
In terms of its definition, the Maxwell-Boltzmann Distribution represents the distribution of molecular energies in gases. It is a plot of the number of particles, where each particle has a particular energy.
What does the Maxwell distribution show?
A Maxwell-Boltzmann Distribution is a probability distribution used for describing the speeds of various particles within a stationary container at a specific temperature. The distribution is often represented with a graph, with the y-axis defined as the number of molecules and the x-axis defined as the speed.
What does the Maxwell-Boltzmann equation represent?
The Maxwell-Boltzmann equation, which forms the basis of the kinetic theory of gases, defines the distribution of speeds for a gas at a certain temperature. From this distribution function, the most probable speed, the average speed, and the root-mean-square speed can be derived.
What is K Maxwell-Boltzmann distribution?
The distribution function for a gas obeying Maxwell-Boltzmann statistics ( fM–B) can be written in terms of the total energy (E) of the system of particles described by the distribution, the absolute temperature (T) of the gas, the Boltzmann constant (k = 1.38 × 10−16 erg per kelvin), and a normalizing constant (C) ...
What are the properties of Maxwell-Boltzmann statistics?
In statistical mechanics, Maxwell–Boltzmann statistics describes the distribution of classical material particles over various energy states in thermal equilibrium. It is applicable when the temperature is high enough or the particle density is low enough to render quantum effects negligible.
How do you read Maxwell-Boltzmann distribution graph?
The Maxwell-Boltzmann distribution is often represented with the following graph. The y-axis of the Maxwell-Boltzmann graph can be thought of as giving the number of molecules per unit speed. So, if the graph is higher in a given region, it means that there are more gas molecules moving with those speeds.
What are the applications of Maxwell-Boltzmann distribution law?
The Boltzmann distribution describes the distribution of energy among classical (distinguishable) particles: It can be used to evaluate the average energy per particle in the circumstance where there is no energy-dependent density of states to skew the distribution.
What are the basic postulates of Maxwell-Boltzmann distribution law?
Maxwell-Boltzmann (MB) Statistics The basic postulates of MB statistics are:- (i)The associated particles are distinguishable. Each energy state can contain any number of particles. (iii)Total number of particles in the entire system is constant. (iv)Total energy of all the particles in the entire system is constant.
Does the Maxwell-Boltzmann distribution only apply to gases?
So the answer to the question asked in your title, "Does the Maxwell-Boltzmann distribution apply to gases only?" is "no"; it applies to all phases of matter, in the sense that it describes the distribution of particle speeds and energies.
What is Boltzmann constant value?
Having dimensions of energy per degree of temperature, the Boltzmann constant has a defined value of 1.380649 × 10−23 joule per kelvin (K), or 1.380649 × 10−16 erg per kelvin.
How is Maxwell-Boltzmann distribution calculated?
Maxwell-Boltzmann Distribution of Speeds f ( v ) = 4 π ( m 2 k B T ) 3 / 2 v 2 e ( − m v 2 / ( 2 k B T ) ) .
How does pressure affect the Maxwell-Boltzmann distribution?
If the reaction is a gas, increasing the pressure of the gas results in the same effect. Raising the pressure causes the particles to be closer together, increasing the number in a given volume.
What are the important features of Maxwell distribution of molecular velocities explain how these can be demonstrated?
(i) The fraction of molecules with very low or high velocities is very small. (ii) The fraction of molecules possessing higher velocities goes on increasing till it reaches the peak and further it starts decreasing. (iii) The maximum fraction of molecules possesses a velocity, corresponding to the peak in the curve.
What are the applications of Maxwell-Boltzmann distribution law?
The Boltzmann distribution describes the distribution of energy among classical (distinguishable) particles: It can be used to evaluate the average energy per particle in the circumstance where there is no energy-dependent density of states to skew the distribution.
What is Maxwell velocity distribution derived from classical statistics?
Maxwell distribution of velocities states that the gaseous molecules inside the system travel at different velocities. Fraction F(v) = 4πN(m2πkT)3/2v2e−mv2/2kT. The Maxwell distribution of velocities can be derived from Boltzmann's equation: f(E)=Ae−kT.
How does pressure affect the Maxwell-Boltzmann distribution?
If the reaction is a gas, increasing the pressure of the gas results in the same effect. Raising the pressure causes the particles to be closer together, increasing the number in a given volume.
Who came up with the Maxwell-Boltzmann distribution theory?
Maxwell Boltzmann Distribution Derivation. The molecules inside the system travel at varying speeds so two persons named James Maxwell and Ludwig Boltzmann came up with a theory to demonstrate how the speeds of the molecule are distributed for an ideal gas which is Maxwell-Boltzmann distribution theory. Consider a system having n particles ...
What is Boltzmann's distribution law?
Ans: Boltzmann's distribution law is a probability measure that gives the probability of a system in a certain state as a function of that state’s energy ε ,and the temperature T of the system.
What is the Maxwell-Boltzmann equation?
It defines the distribution of speeds for gas molecules at a certain temperature. From this distribution function, the most probable speed, CAverage, and Crms can be derived.
What is Maxwell distribution?
Maxwell distribution of velocities states that the gaseous molecules inside the system travel at different velocities.
How does the Maxwell-Boltzmann Distribution work?
The formula for the Maxwell-Boltzmann Distribution provides the probability density as a function of the speed of the molecule. The probability density depends on the temperature of the gas (
Why is the average of a graph offset to the right of the peak?
Because the tail of the graph extends further on the right of the peak than the left, it makes sense that the average would be offset to the right of the distribution's peak. The total area underneath the distribution curve equates to the total number of molecules in the gas.
Maxwell Boltzmann Distribution Derivation
Maxwell Boltzmann Distribution Equation Derivation
- Maxwell distribution of velocities states that the gaseous molecules inside the system travel at different velocities. Fraction F(v) = 4πN(m2πkT)3/2v2e−mv2/2kT The Maxwell distribution of velocities can be derived from Boltzmann’s equation: f(E)=Ae−kT This equation tells us the probability that a molecule will be found with energy E that decreases ...
Maxwell Boltzmann Equation Derivation
- The probability of finding a molecule at some velocity is one. Then, by using the definite integral form here from minus-infinity to plus-infinity as: ∫−∞∞e−x2dx=π and substituting x=m2kTvz Am2kTvz∫−∞∞e−m2πkTdx=π F(vz)=m2kTv2e−mvz2/2kT m2kTdvz=1 It gives A=m2kT This normalizes the function to: F(vz)=m2kTv2e−mvz2/2kT In terms of three dimensions, it becomes…
Maxwell Boltzmann Derivation
- Consider the fraction of molecules in a three-dimensional box having the translation energy ε, then, as a function, it will be: ε = h2/ 8m [nx2+ ny2+ nz2]/[Lx2+ Ly2+ Lz2] ni2= nx2+ ny2+ nz2 ,and εi = n2h2/8mL2 The length of each side of the box is equal. So, we would consider the density of translational states as a sphere in space. So the total volume of the sphere is: ϵ=4π8∫…