
What do the numbers next to the element ions mean?
The number next to the simbol of the element ions (as a superscript) means the number of charges of the ion. For example N (+),, where (+) is a superscript means that the charge of the ion is 1+. S(2-), where (2-) is a superscript, means that the charge of the ion is (2-). OH (-), where (-) is a superscript, means that the charge of OH ion is (1-).
What does the number next to an atom signify?
Atoms of the same element that have different numbers of neutrons What does the number next to the ion signify? The charge of the atom. What does the number next to an isotope signify when you see it written like this Carbon-14 The number represents the mass (protons plus neutrons)
What does the number next to an isotope mean?
The charge of the atom. What does the number next to an isotope signify when you see it written like this Carbon-14 The number represents the mass (protons plus neutrons) How can you tell isotopes apart? The mass number is different than what you see on the periodic table.
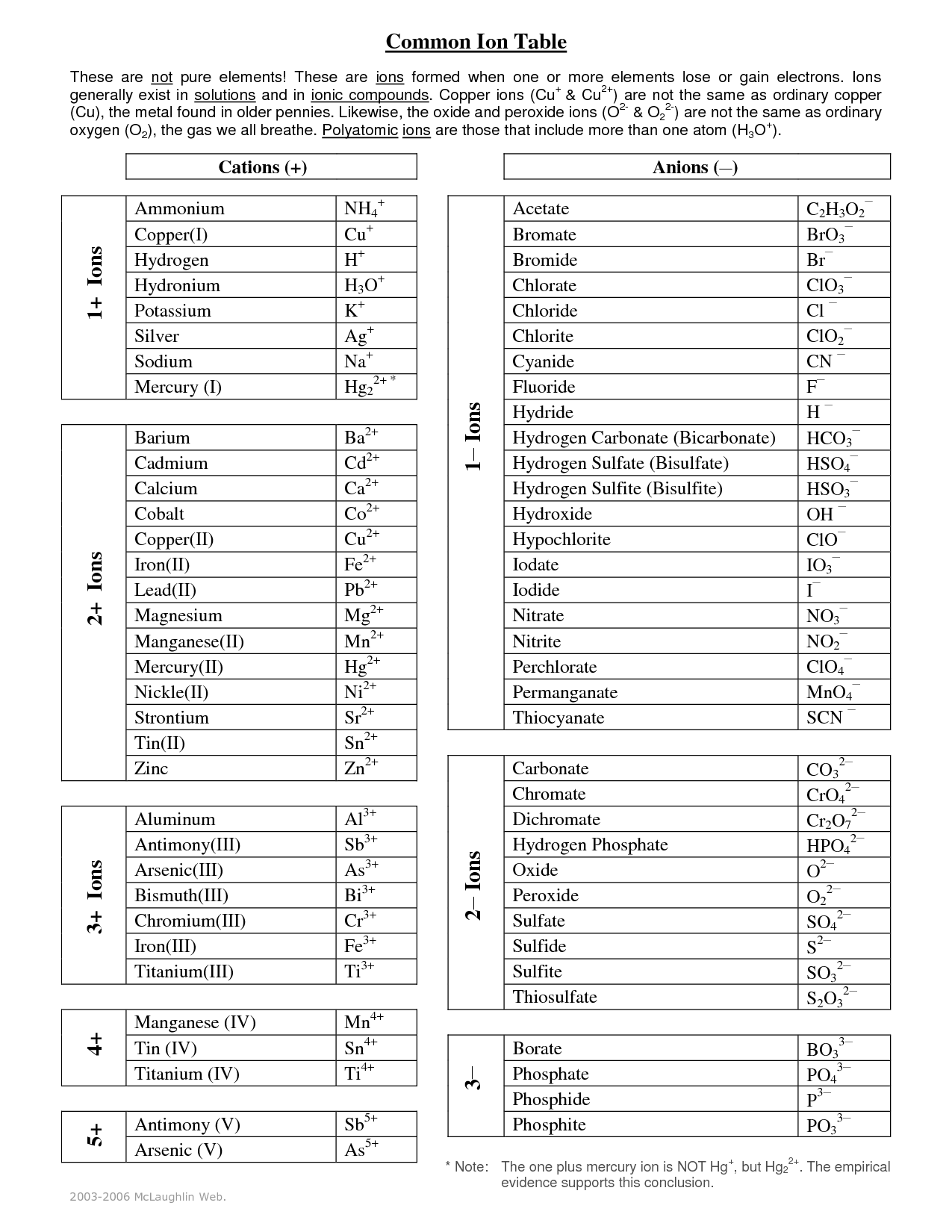
What do the numbers next to ions mean?
Charge numbers in chemistry. Charge number or valence of an ion is the coefficient that, when multiplied by the elementary charge, gives the ion's charge. For example, the charge on a chloride ion, , is , where e is the elementary charge. This means that the charge number for the ion is .
What does 2+ mean in ions?
If only a "+" is present, it means the charge is +1. For example, Ca2+ indicates a cation with a +2 charge. Anions are ions that carry a net negative charge. In anions, there are more electrons than protons.
What is an ion number?
This number is the atomic number of the element. A positively-charged ion or cation has more protons than electrons. The proton number is the atomic number of the element, while the electron number is the atomic number minus the charge. A negatively-charged ion or anion has more electrons than protons.
What does 2+ mean in chemistry?
Even though we use the positive and negative symbols for charge, they are not positive and negative numbers. In the context of charges, 2+ means two positive things, 3- means three negative things.
How do you read ions?
When writing the symbol for an ion, the one- or two-letter element symbol is written first, followed by a superscript. The superscript has the number of charges on the ion followed by a + (for positive ions or cations) or - (for negative ions or anions). Neutral atoms have a charge of zero, so no superscript is given.
What's the difference between +2 and 2+ in chemistry?
No difference …. We use the plus sign just to indicate the number is positive… while certain concepts or theories may require you to put a '+' sign… 2 and +2 are same … as the convention suggests… a number without any precceding sign is considered to be a positive number ….
Do ions have different number of electrons?
An ion is an atom or group of atoms in which the number of electron s is different from the number of proton s. If the number of electrons is less than the number of protons, the particle is a positive ion, also called a cation.
Are ions positive or negative?
ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Positively charged ions are called cations; negatively charged ions, anions.
How do you know if an ion is positive or negative?
If the atom has more electrons than protons, it is a negative ion, or ANION. If it has more protons than electrons,it is a positive ion.
What does the 1 in 1s stand for?
The orbital occupied by the hydrogen electron is called a 1s orbital. The "1" represents the fact that the orbital is in the energy level closest to the nucleus. The "s" tells you about the shape of the orbital.
What does a charge of 2+ mean?
+2 charge indicates that any compound, molecule, or atom loses two electrons and becomes a cationic species. -2 charge indicates that any compound, molecule, or atom loses two electrons and becomes an anionic species.
What does the superscript 2+ mean in ca2+?
If a charge is present, it's indicated in superscript, with a sign (+/-) and a number if more than one charge is present. For example, calcium ions have two positive charges so are written Ca2+.
What ion has a charge of 2?
A magnesium atom must lose two electrons to have the same number electrons as an atom of the previous noble gas, neon. Thus, a magnesium atom will form a cation with two fewer electrons than protons and a charge of 2+. The symbol for the ion is Mg2+, and it is called a magnesium ion.
Which will form an ion with a 2 charge?
alkaline earth metalsThe alkaline earth metals (red) always form +2 ions.
What called mg2+?
magnesium(II) cation.
Which is the correct name for the Mg +2 ion?
Magnesium ionMagnesium ion | Mg+2 - PubChem.