What is the plum pudding model of atom?
The plum pudding model is an obsolete scientific model of the atom proposed by J. J. Thomson in 1904. It was devised shortly after the discovery of the electron but before the discovery of the atomic nucleus.
Why is it called plum pudding?
The colloquial nickname "plum pudding" was soon attributed to Thomson's model as the distribution of electrons within its positively-charged region of space reminded many scientists of plums in the common English dessert, plum pudding. In 1909, Hans Geiger and Ernest Marsden conducted experiments with thin sheets of gold.
What happened to Thomson’s plum pudding model?
Thomson’s Plum Pudding Model hold sway for few years until the Ernest Rutherford announced the nuclear model of the atom in the year of 1911. After Ernest Rutherford’s nuclear model of the atom in 1911, the interest in Thomson’s Plum Pudding Model fell off rapidly.
When was the plum pudding model published?
Thomson Plum Pudding Model (1911) Published on 24/2/2012 and Updated on 25/8/2018. Thomson's Plum Pudding Model is given by the English Physicist Sir Joseph John J.J. Thomson. He discovered the electron (first subatomic particle) in the year of 1897.
What does the plum pudding represent?
Thomson's Plum Pudding Model is the first model to represent the atomic structure of matter. The positively charged sphere exerts the force on negatively charged electrons. The direction of the net force on negatively charged electrons due to positively charged sphere is towards the center of the sphere.
What does the pudding represent in the plum pudding model of the atom?
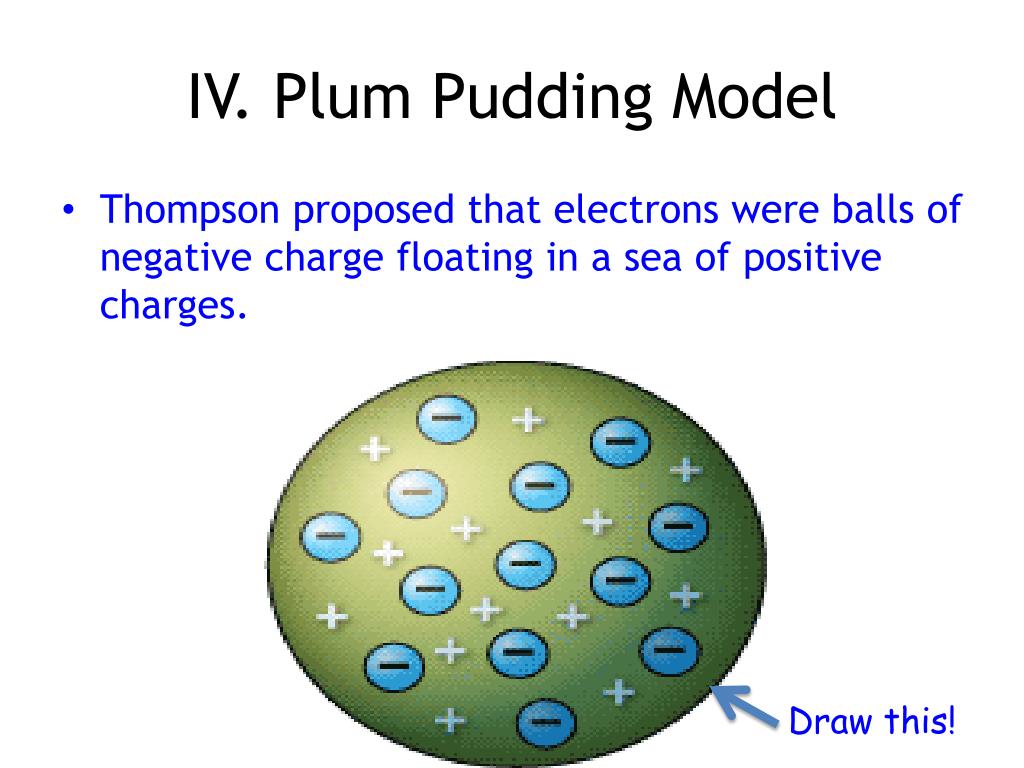
According to this model, the atom is a sphere of positive charge, and negatively charged electrons are embedded in it to balance the total positive charge. The electrons are like plums in a pudding.
What are the main ideas of the plum pudding model?
The plum pudding model After discovering the electron in 1897, J J Thomson proposed that the atom looked like a plum pudding . To explain the two types of static electricity, he suggested that the atom consisted of positive 'dough' with a lot of negative electrons stuck in it.
What is an example of plum pudding model?
Plum pudding is an English dessert similar to a blueberry muffin. In Thomson's plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge, like blueberries stuck into a muffin. The positive matter was thought to be jelly-like, or similar to a thick soup.
Which of the following statements best describes the plum pudding model of atom?
According to the Plum-Pudding model, an atom is composed of negatively charged electrons distributed in a uniformly positively charged volume of the atom just like plums are distributed in a pudding.
Why is it comparable to a plum pudding or raisin bread?
Description of his model: Thomson's model was known as the "Plum Pudding Model” (or "Raisin Bread Model.") As each atom was a sphere filled with a positively charged fluid, known as the “pudding”. Scattered in this fluid were negatively charged electrons, these were the “plums” in the pudding.
Why was the plum pudding model flawed?
He argued that the plum pudding model was incorrect. The symmetrical distribution of charge would allow all the α particles to pass through with no deflection. Rutherford proposed that the atom is mostly empty space. The electrons revolve in circular orbits about a massive positive charge at the centre.
What did the raisins plums in the plum pudding cake represent?
Explanation: The plums represented the electrons and the pudding represented the positively charged part of the atom in which the electrons were suspended.
Why did the plum pudding model fail?
∙ It failed to explain the concept of nucleus of an atom, which was later on discovered by Rutherford. Rutherford also discovered that the electrons revolve around the nucleus, which was not explained in the plum pudding model.
How was the plum pudding model disproved?
Ernest Rutherford discovered the atomic nucleus using a cathode ray tube. When alpha particles are fired at a thin gold foil, they never go through. Ernest Rutherford proved that the plum-pudding model was incorrect. Ernest Rutherford experimented by firing cathode rays at gold foil.
Why was Thomson's model of atom failed?
Thomson model of an atom could not explain the stability of an atom, i.e., how a positive charge in the atom holds the negatively charged electrons. It could not explain the position of the nucleus in an atom and the scattering of alpha particles.
Is the plum pudding model accurate?
Thompson's plum pudding model described a positive medium (pudding) with negatively charged particles dispersed throughout (plums). This model we know now is not correct, but it was used as the basis for other scientists' models of atoms.
Why was the plum pudding model of the atom rejected?
Thomson suggested the atom's plum pudding model, which had negatively charged electrons trapped in a "soup" filled with positive effects. Thomson was "wrong" because he assumed that the positive charge of an atom was all one uniform blob of matter with the electrons moving in orbits inside the nucleus.
Who is the proponent of the plum pudding model of an atom?
Thomson was initially a strong proponent of what is commonly called the plum-pudding atomic model or the Thomson atomic model, although many other representations of the atom were suggested by his contemporaries.
How is the plum pudding model different to the nuclear model?
Summary. The plum pudding model is an early attempt to show what an atom looks like. Bombardment of gold foil with alpha particles showed that some particles were deflected. The nuclear model of the atom consists of a small and dense positively charged interior surrounded by a cloud of electrons.
Why Thomson's atomic model is also known as plum pudding model How do you know that nucleus is very small as compared to the size of atom?
Thomson's Atomic Model- Postulates Thomson's atomic model resembles a spherical plum pudding as well as a watermelon. It resembles a plum pudding because the electrons in the model look like the dry fruits embedded in a sphere of positive charge just like a spherical plum pudding.
What is the Plum Pudding Model?
Plum pudding model. The plum pudding model is one of several historical scientific models of the atom. First proposed by J. J. Thomson in 1904 soon after the discovery of the electron, but before the discovery of the atomic nucleus, the model tried to explain two properties of atoms then known: that electrons are negatively charged particles ...
What is the Thomson problem in the Plum Pudding model?
The Thomson problem is a natural consequence of the plum pudding model in the absence of its uniform positive background charge.
What is the charge of a plum pudding?
The plum pudding model has electrons surrounded by a volume of positive charge, like negatively charged "plums" embedded in a positively charged " pudding ".
Why did Thomson propose a positive volume charge?
His proposal of a positive volume charge reflects the nature of his scientific approach to discovery which was to propose ideas to guide future experiments.
When was the Thomson model proposed?
Thomson published his proposed model in the March 1904 edition of the Philosophical Magazine, the leading British science journal of the day. In Thomson's view: ... the atoms of the elements consist of a number of negatively electrified corpuscles enclosed in a sphere of uniform positive electrification, ...
Who was the professor of the gold atom experiment?
Their professor, Ernest Rutherford, expected to find results consistent with Thomson's atomic model. It was not until 1911 that Rutherford correctly interpreted the experiment's results which implied the presence of a very small nucleus of positive charge at the center of each gold atom.
Who developed the Rutherford model?
This led to the development of the Rutherford model of the atom. Immediately after Rutherford published his results, Antonius Van den Broek made the intuitive proposal that the atomic number of an atom is the total number of units of charge present in its nucleus.
What was the significance of the Plum Pudding Model?
His work in determining that atom’s were divisible, as well as the existence of electromagnetic forces within the atom, would also prove to be major influence on the field of quantum physics.
Why is the Plum Pudding Model important?
Though defunct by modern standards, the Plum Pudding Model represents an important step in the development of atomic theory. Not only did it incorporate new discoveries, such as the existence of the electron, it also introduced the notion of the atom as a non-inert, divisible mass. Henceforth, scientists would understand that atoms were themselves composed of smaller units of matter and that all atoms interacted with each other through many different forces.
Why is plum pudding called plum pudding?
And from this, the Plum Pudding Model was born, so named because it closely resembled the English desert that consists of plum cake and raisins. The concept was introduced to the world in the March 1904 edition of the UK’s Philosophical Magazine, to wide acclaim.
What episode of Astronomy Cast is the standard model?
Astronomy Cast also has some episodes on the subject: Episode 138: Quantum Mechanics, Episode 139: Energy Levels and Spectra, Episode 378: Rutherford and Atoms and Episode 392: The Standard Model – Intro.
When was the atomic model first proposed?
Ever since it was first proposed by Democritus in the 5th century BCE, the atomic model has gone through several refinements over the past few thousand years. From its humble beginnings as an inert, indivisible solid that interacts mechanically with other atoms, ongoing research and improved methods have led scientists to conclude ...
Which model of the atom is surrounded by an equal number of electrons in orbital shells?
the Bohr Model ).
What is the watermelon model of atom?
This model is also called as watermelon model of atom because it resembles a spherical plum pudding as well as a watermelon.
Why was Thomson's model correct?
At the time, Thomson's model was correct, because it explained everything scientists already understood about the atom. Thomson 's model was dismissed by the Japanese physicist Hantaro Nagaoka. He said a massive nucleus was in the atom. The electrons, like the rings revolving around Saturn, revolved around the nucleus.
What did Rutherford prove about the Thomson hypothesis?
Rutherford has shown the atom has a small, massive, positively charged nucleus in it. He has also confirmed with Nagaoka that the electrons move outside the nucleus in circular orbits.
What was the goal of the atomic model?
The goal of each atomic model was to present all the experimental evidence of atoms in the simplest way possible. Thomson proposed the atom should be composed of electrons surrounded by a broth of positive charge to counteract the negative charges of the electrons. Which led to plum pudding model.
What is the red part of a watermelon compared to?
The model was also compared with a watermelon because the red edible part of a watermelon was compared to the positively charged sphere and the black seeds filling the watermelon looked similar to the electrons inside the sphere. An atom consists of a positively charged sphere, and it embeds the electrons.
Who invented the electron model?
Plum Pudding Model. J. J. Thomson, who invented the electron in the year 1897, suggested the atom's plum pudding model in 1904 before the atomic nucleus was found, to include the electron in the atomic model. In this model, the atom consists of electrons (which Thomson called them "corpuscles") surrounded by a soup of positive charge ...
Which model fails to explain Rutherford's -particle scattering experiment?
Thomson's atom model fails to explain Rutherford's α-particle scattering experiment in which most of the fast moving α - particles went through the gold foil straight away.
Answer
the answer is According to this model, the atom is a sphere of positive charge, and negatively charged electrons are embedded in it to balance the total positive charge. The electrons are like plums in a pudding.
New questions in Chemistry
What conclusion can be drawn about the number of elements the make up the majority of the world in which we live and the total number of elements iden …
