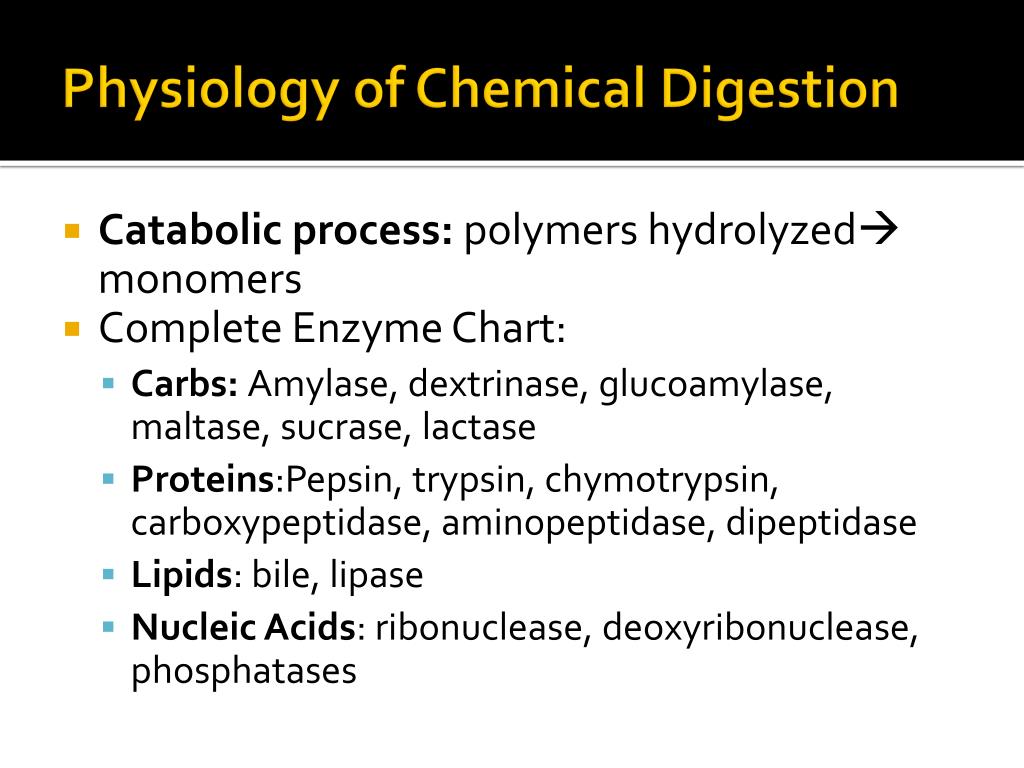
What does trypsin do in chemical digestion?
Trypsin is an enzyme that helps us digest protein. In the small intestine, trypsin breaks down proteins, continuing the process of digestion that began in the stomach. It may also be referred to as a proteolytic enzyme, or proteinase. Trypsin is produced by the pancreas in an inactive form called trypsinogen.
What is the function of the trypsin in the body?
- Producing hormones
- Potentiating muscle growth
- Repairing tissue (including skin, muscles, bones, cartilage, and blood)
- Building neurotransmitters in the brain
What does pepsin do in digestion?
Pepsin is an enzyme in the stomach that helps break down the protein in your food for digestion. Specifically, it acts on the proteins in meat, eggs, dairy products, nuts, and seeds. It is the first enzyme to attack protein in a group of enzymes known as proteases (you’ll often see this term on digestion supplement labels).
What is the primary function of the digestion system?
What are the 5 main functions of the digestive system?
- Ingestion of food.
- Secretion of fluids and digestive enzymes.
- Mixing and movement of food and wastes through the body.
- Digestion of food into smaller pieces.
- Absorption of nutrients.
- Excretion of wastes.

How does trypsin break down proteins?
Trypsin breaks down proteins via a catalytic reaction called hydrolysis. Hydrolysis occurs when a chemical compound is broken down in the presence...
What is trypsin and its function?
Trypsin is a digestive enzyme that is secreted from the pancreas and then migrates to the small intestine. It breaks down proteins to facilitate di...
Where trypsin is secreted?
Trypsin is secreted from the pancreas in its inactive form, trypsinogen. Trypsinogen migrates to the small intestine and is then activated or cleav...
What is the function of trypsin?
It’s precursor (trypsinogen) is produced by the pancreas and its primary function is to digest proteins. 1 The breakdown of proteins by trypsin starts in the small intestine as trypsinogen (the inactive form of trypsin) travels from the pancreas to the small intestine and is then converted to trypsin.
What Is Trypsin Used For?
There is not enough evidence to back the claims that trypsin is effective for many conditions, including:
How much trypsin is in a pill?
The average oral dose of trypsin is up to 50 milligrams (mg) and is most often combined with bromelain (another proteolytic enzyme ). 4
What is the function of trypsin and chymotrypsin?
Trypsin (also sometimes referred to as a proteinase) goes to work with two other proteinases called pepsin and chymotrypsin to break down protein (from food) into amino acids. Amino acids are building blocks of protein and they are used in the body for many functions, including: Producing hormones.
What does high trypsin levels indicate?
Note: high levels of immunoreactive trypsin (IRT) in babies may indicate the presence of genes for the recessive genetic disorder, cystic fibrosis. 3
What happens when the body doesn't produce enough trypsin?
When the body doesn’t produce enough trypsin, it can lead to a condition called malabsorption. Malabsorption is the decreased ability of the body to digest and absorb an adequate supply of nutrients.
Where does trypsinogen come from?
It’s precursor (trypsinogen) is produced by the pancreas and its primary function is to digest proteins. 1 The breakdown of proteins by trypsin starts in the small intestine as trypsinogen (the inactive form of trypsin) travels from the pancreas to the small intestine and is then converted to trypsin. Trypsin (also sometimes referred ...
Where does trypsin go?
Trypsin is a digestive enzyme that is secreted from the pancreas and then migrates to the small intestine. It breaks down proteins to facilitate digestion.
How does trypsin cleave proteins?
Like all other serine proteases, trypsin cleaves proteins by using a hydrolysis reaction. A hydrolysis reaction is a general catalytic mechanism where a bond is cleaved in the presence of water. Recall that a catalyst lowers the activation energy to increase the rate of a product. Trypsin's catalytic mechanism contains the following steps:
What is the enzyme that breaks down proteins into smaller peptides?
Trypsin is an enzyme that breaks down large proteins into smaller peptides. Enzymes lower the activation energy of a reaction and allow for more products to be made. Peptides are strands of two or more amino acids held together by peptide bonds. Any enzyme that breaks peptide bonds is called a proteolytic enzyme. Trypsin also belongs to a family of proteins called serine proteases. They get this name because they all have the amino acid serine in their active site. An active site is the specific portion of a molecule that is responsible for catalyzing a reaction. Trypsin is secreted from the pancreas as a zymogen (an inactive enzyme) by the name of trypsinogen. Once trypsinogen moves from the pancreas to the small intestine, it is cleaved and released as the active enzyme trypsin .
What is the enzyme that breaks down proteins?
Trypsin is a digestive enzyme that breaks down protein in the gut. An enzyme is a catalyst protein that increases the speed of a chemical reaction by lowering the activation energy. Activation energy is the energy required for a reaction to begin. Trypsin is secreted by the pancreas in its inactive form and then is activated once it migrates to the small intestine. It belongs to a family of enzymes called serine proteases. These proteases are enzymes that break down proteins and contain the amino acid serine at its active site. An active site is the specific region of a molecule where a reaction occurs. Proteins are made up of amino acids linked together by peptide bonds. Trypsin cleaves peptide bonds to turn one large protein into smaller pieces. Even though trypsin belongs to the serine protease family, it is also called a proteolytic enzyme, or an enzyme that breaks peptide bonds.
What is the inactive form of trypsin?
The pancreas secretes the inactive form of trypsin called trypsinogen. An inactive enzyme that must be activated by another enzyme is called a zymogen. The zymo gen trypsinogen remains inactive to prevent any cleaving from happening in the pancreas. Once trypsinogen migrates to the small intestine, it is cleaved and activated to become trypsin. After activation, trypsin is ready and available to break down dietary protein. Active trypsin molecules in the small intestine have the ability to activate incoming trypsinogen as well as other pancreatic zymogens. This includes the activation of:
What is the hydroxyl group of trypsin?
The serine in trypsin's active site has a hydroxyl group (-OH) that loses a hydrogen to a nearby histidine in the same active site. The remaining oxygen is now available to bind to the substrate (the larger protein that trypsin will eventually break down into peptides) by forming a covalent bond with the substrate's carbonyl (C=O) carbon.
What is the pH of trypsin?
Trypsin has a theoretical molecular weight of 23.3 kDa. It operates within a pH range of 7.5-8.5 and an optimal temperature of 37 degrees Celcius (98.6 degrees Fahrenheit).
How does trypsin work?
The way trypsin works is by hydrolysis or the complete separation of proteins into parts that are highly nutritious to the health of the human body. Trypsin is naturally produced in the pancreas and once needed it is secreted by the pancreas into the small intestine and gets to work on the hydrolysis. What the hydrolysis process does well is converting proteins into peptides and then converting peptides into amino acids which is the purest and best form of protein for the body.
Why is trypsin important?
Trypsin is one of the more needed and important essential enzymes for a healthy digestive system and even healthier body as well. As we mentioned earlier, trypsin is a pillar of the enzymes that help break down proteins along with pepsin, protease and peptidase. Finding the right blend of enzymes to better help your digestive system is something that we all can achieve. There’s no reason to hold off on eating something because you’re worried about the outcome. All foods have nutrients we need and not giving our bodies all the options available isn’t smart.
What is the role of trypsin in the breakdown of proteins?
Trypsin is considered one of the pillars of a group of enzymes in our bodies that breaks down proteins into essential nutrients and ready for absorption by the small intestine.
Can you supplement your body with enzymes?
If you decide to supplement your body with certain enzymes, it is very important to choose a product that has very pure ingredients and will show results. Take a look at our top ranked digestive enzyme blend and see how it can help your digestive issues.
Is trypsin good for digestion?
Besides the benefits from trypsin as far as digestion go, trypsin has been found to be beneficial in the regeneration or harvesting of skin cells. The use of trypsin as a supplement is not uncommon for people looking to maximize the absorption of amino acids into their bodies. Getting the most out of food is something that every adult is conscious of but takes a while to get around to. Little by little, digestive health issues will become apparent and to not do anything about it is wrong.
What is trypsin enzyme?
Show bio. Laura has a Masters of Science in Food Science and Human Nutrition and has taught college Science. Trypsin is one of the enzymes used to digest proteins. It is very similar to another protein digestion enzyme, chymotrypsin.
What enzyme breaks down protein?
One of the most studied enzymes is chymotrypsin, but another protein digestion enzyme is trypsin . Chymotrypsin and trypsin are very similar enzymes, thus when studying trypsin it is often in comparison ...
What enzyme breaks peptide bonds?
Trypsin is one of the enzymes used to digest proteins. It breaks the peptide bonds at the C terminal of the basic amino acids, lysine and arginine. Trypsin is very similar to another protein digestion enzyme, chymotrypsin.
What is the specificity pocket of chymotrypsin?
In order to accomplish this, the specificity pocket of chymotrypsin includes a serine at position 189 while trypsin contains an aspartic acid in that position. This seems like a very small difference, but serine is not polar, thus it will allow hydrophobic amino acids into the pocket.
How does hydrogen on serine work?
The mechanism is the exactly the same for the two molecules as well, with four steps: Hydrogen on serine is removed which attacks the carbon on the peptide bond. Carbon-oxygen double bond reforms, kicking off the nitrogen on the peptide, releasing the first half of the protein.
Why is the peptide bond broken but the reaction isn't done?
The peptide bond was broken but the reaction isn't done because the other half of the protein (the section with the basic amino acid) is still attached to the enzyme. The last two steps are in order to help the enzyme release this second half of the protein. In order to accomplish this, water is added.
Which amino acid cleaves the protein at the C-terminal?
Chymotrypsin cleaves the protein at the c-terminal of aromatic amino acids while trypsin cleaves the protein at the c-terminal of the basic amino acids lysine and arginine. Lesson. Quiz. Course.
Why is trypsin used in vitro?
Some cell types adhere to the sides and bottom of a dish when cultivated in vitro. Trypsin is used to cleave proteins holding the cultured cells to the dish , so that the cells can be removed from the plates. Trypsin can also be used to dissociate dissected cells (for example, prior to cell fixing and sorting).
Where is trypsin found?
Trypsin ( EC 3.4.21.4) is a serine protease from the PA clan superfamily, found in the digestive system of many vertebrates, where it hydrolyzes proteins. Trypsin is formed in the small intestine when its proenzyme form, the trypsinogen produced by the pancreas, is activated.
What is the optimal temperature for trypsin?
Human trypsin has an optimal operating temperature of about 37 °C. In contrast, the Atlantic cod has several types of trypsins for the poikilotherm fish to survive at different body temperatures. Cod trypsins include trypsin I with an activity range of 4 to 65 °C (40 to 150 °F) and maximal activity at 55 °C (130 °F), as well as trypsin Y with a range of 2 to 30 °C (36 to 86 °F) and a maximal activity at 21 °C (70 °F).
How cold should trypsin be stored?
Trypsin should be stored at very cold temperatures (between −20 and −80 °C) to prevent autolysis, which may also be impeded by storage of trypsin at pH 3 or by using trypsin modified by reductive methylation. When the pH is adjusted back to pH 8, activity returns.
How much does trypsin weigh?
As a protein, trypsin has various molecular weights depending on the source. For example, a molecular weight of 23.3 kDa is reported for trypsin from bovine and porcine sources.
What is the process of converting peptides into amino acids?
In the duodenum, trypsin catalyzes the hydrolysis of peptide bonds, breaking down proteins into smaller peptides. The peptide products are then further hydrolyzed into amino acids via other proteases, rendering them available for absorption into the blood stream. Tryptic digestion is a necessary step in protein absorption, as proteins are generally too large to be absorbed through the lining of the small intestine.
When was trypsin discovered?
Trypsin was discovered in 1876 by Wilhelm Kühne and was named from the Ancient Greek word for rubbing since it was first isolated by rubbing the pancreas with glycerin. Trypsin. Identifiers. EC no.
