What is the atomic mass unit?
When is the atomic mass of all elements 2021?

What has a atomic number of 89?
actinium (Ac), radioactive chemical element, in Group 3 (IIIb) of the periodic table, atomic number 89.
What is element 89 in the periodic table?
ActiniumActinium, 89 on the periodic table, belongs to a group of elements also known as the rare-earth elements. It is a group 3 element and the first in a series of elements known as the actinides.
What element has an atomic number of 39 and a mass number of 89?
YttriumYttrium is a chemical element with symbol Y and atomic number 39. Classified as a transition metal, Yttrium is a solid at room temperature.
What has a mass number of 88?
strontiumThe mass number of strontium is 88.
What is the charge of a nucleus of element 89?
+89It has this name because the first element in the series is actinium (Ac) with an atomic number of 89. The number indicates that every nucleus of an actinium atom has 89 protons. Therefore, the charge of an actinium atom nucleus must be +89.
Who is radium?
Radium was discovered by Marie Skłodowska-Curie and her husband Pierre Curie on 21 December 1898, in a uraninite (pitchblende) sample from Jáchymov. While studying the mineral earlier, the Curies removed uranium from it and found that the remaining material was still radioactive.
How do you find the mass number of an element?
Together, the number of protons and the number of neutrons determine an element's mass number: mass number = protons + neutrons. If you want to calculate how many neutrons an atom has, you can simply subtract the number of protons, or atomic number, from the mass number.
What element has a mass number of 90 and 39 electrons?
Atomic Number of Yttrium Yttrium is a chemical element with atomic number 39 which means there are 39 protons and 39 electrons in the atomic structure. The chemical symbol for Yttrium is Y.
What is the mass number of all elements?
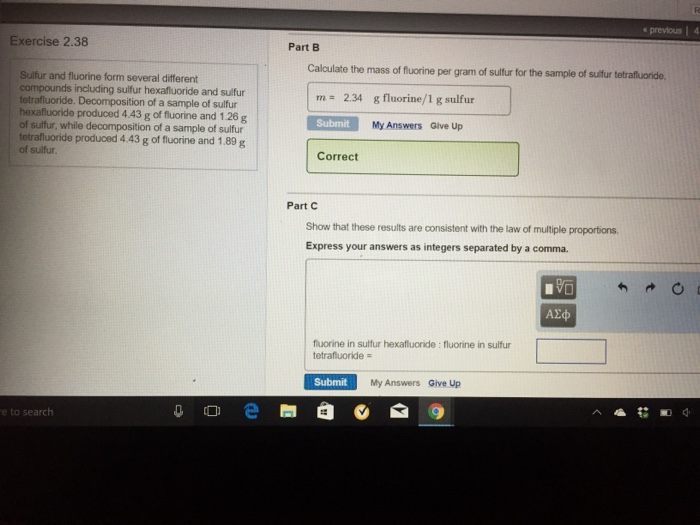
The mass number is defined as the total number of protons and neutrons in an atom. It can be calculated by adding the number of neutrons and the number of protons (atomic number) together. Consider Table 4.17. 1 below that shows data from the first six elements of the periodic table.
How many neutrons are there in 88?
Number of neutrons = 88 - 38 = 50. Was this answer helpful?
Has an atomic number of 86?
RadonRadon is element 86 in the periodic table and is one of the noble gases along with helium, neon, argon, krypton and xenon.
What has a mass number of 119?
Ununennium, also known as eka-francium or element 119, is the hypothetical chemical element with symbol Uue and atomic number 119. Ununennium and Uue are the temporary systematic IUPAC name and symbol respectively, which are used until the element is discovered, confirmed, and a permanent name is decided upon.
Are there 127 elements?
As of April 2022, synthesis has been attempted for every element up to and including unbiseptium (Z = 127), except unbitrium (Z = 123), with the heaviest successfully synthesized element being oganesson in 2002 and the most recent discovery being that of tennessine in 2010.
What is zirconium used for?
It is used to make crucibles that will withstand heat-shock, furnace linings, foundry bricks, abrasives and by the glass and ceramics industries. It is so strong that even scissors and knives can be made from it. It is also used in cosmetics, antiperspirants, food packaging and to make microwave filters.
What is the 128 element?
Please visit the Tellurium element page for information specific to the chemical element of the periodic table.
What color is Lithium?
silvery-whiteLithium is a soft, silvery-white, metal that heads group 1, the alkali metals group, of the periodic table of the elements. It reacts vigorously with water.
What is the atomic mass of an atom?
The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. The atomic mass is carried by the atomic nucleus, which occupies only about 10 -12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance.
What is the mass number of Yttrium?
Mass numbers of typical isotopes of Yttrium are 89.
How many protons does yttrium have?
Yttrium is a chemical element with atomic number 39 which means there are 39 protons and 39 electrons in the atomic structure. The chemical symbol for Yttrium is Y.
How are atomic nuclei determined?
Properties of atomic nuclei (atomic mass, nuclear cross-sections) are determined by the number of protons and number of neutrons (neutron number). It must be noted, especially nuclear cross-sections may vary by many orders from nuclide with the neutron number N to nuclide with the neutron number N+1. For example, actinides with odd neutron number are usually fissile (fissionable with slow neutrons) while actinides with even neutron number are usually not fissile (but are fissionable with fast neutrons). Heavy nuclei with an even number of protons and an even number of neutrons are (due to Pauli exclusion principle) very stable thanks to the occurrence of ‘paired spin’. On the other hand, nuclei with an odd number of protons and neutrons are mostly unstable.
How to determine the stability of an isotope?
To determine the stability of an isotope you can use the ratio neutron/proton (N/Z). Also to help understand this concept there is a chart of the nuclides, known as a Segre chart. This chart shows a plot of the known nuclides as a function of their atomic and neutron numbers. It can be observed from the chart that there are more neutrons than protons in nuclides with Z greater than about 20 (Calcium). These extra neutrons are necessary for stability of the heavier nuclei. The excess neutrons act somewhat like nuclear glue. Only two stable nuclides have fewer neutrons than protons: hydrogen-1 and helium-3.
What is the unit of measure for mass?
The unit of measure for mass is the atomic mass unit (amu). One atomic mass unit is equal to 1.66 x 10 -24 grams. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.
Which is heavier, a proton or a neutron?
The neutron is slightly heavier than the proton. This increases the mass of nuclei with more neutrons than protons relative to the atomic mass unit scale based on 12 C with equal numbers of protons and neutrons.
What is the atomic number of strontium?
Based on the periodic table, the atomic number 38 is for strontium element, and the sign of strontium is Sr. Hence, the element X is Sr.
How many neutrons does strontium have?
The element is strontium and the number of neutrons it have is 51.
What is the atomic mass unit?
The Atomic masses are represented in the Atomic mass unit (u). The elements whose atomic masses are written in bracket ( ) are the synthetic elements and their atomic masses values represent the Atomic Mass of the most stable isotope.
When is the atomic mass of all elements 2021?
Atomic Mass of all Elements (Chart + Rounded values) June 10, 2021 March 7, 2021 by Admin. Atomic mass of all elements (along with the rounded off values) is mentioned in the chart below. Atomic Number.