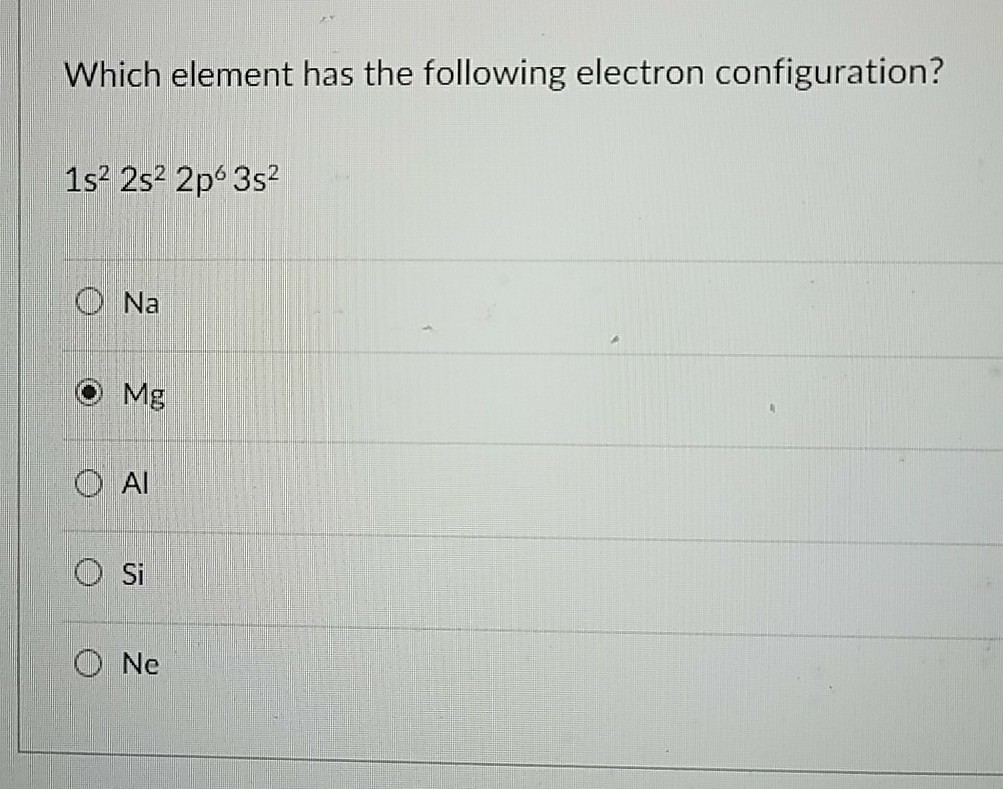
Which element has the strongest electron affinity?
The chemical symbol for Chlorine is Cl. Chlorine is a yellow-green gas at room temperature. It is an extremely reactive element and a strong oxidising agent: among the elements, it has the highest electron affinity and the third-highest electronegativity, behind only oxygen and fluorine.
What does an electron affinity with a negative value indicate?
When an electron is added to a gaseous atom, a change in energy is observed called electron affinity. Electron affinity measures the ease of gaining an electron by an atom. For example, the electron affinity of chlorine is −348.6 kJ/mol. The negative sign indicates that it is an exothermic change.
Is it ever worth it to have negative affinity?
Yes, since its too easy to negate the negative affinity later on. Even if you do not have the skills to get rid of the affinity its still worth it since it compensates with very high raw damage. -affinity on weapon its like an element that will proc sometimes when crit u will see blue arrow down that mean the - affinity reduced the crit dmg.
What elements have no affinity for electrons?
Which elements have no affinity for electrons? Well, noble gases such as Helium, Neon and Argon have an electron affinity nearly zero because they have octet configuration. Click to see full answer. Subsequently, one may also ask, what are the exceptions to electron affinity?
See more

What does most negative electron affinity mean?
Unlike electronegativity, electron affinity is a quantitative measurement of the energy change that occurs when an electron is added to a neutral gas atom. The more negative the electron affinity value, the higher an atom's affinity for electrons.
Which element has the most negative electron affinity quizlet?
Therefore carbon has the highest (most negative) electron affinity rather than nitrogen.
Which element has the most electron affinity?
ChlorineChlorine has the highest electron affinity among the elements. Its high affinity can be attributed to its large atomic radius, or size. Because chlorine's outermost orbital is 3p, its electrons have a large amount of space to share with an incoming electron.
Can you have a negative electron affinity?
So energy is released when an electron is added, and that is because the electron that we added is still able to be attracted to the charged nucleus. So if the nucleus has an attraction for the added electron, you're going to get a negative value for the electron affinity.
Who has the lowest electron affinity?
Thus, oxygen has the lowest electron affinity.
Why does group 17 have the most negative values for electron affinity?
The halogen have a strong tendency to accept electrons due to their small size and effective nuclear charge so they have maximum negative electron gain enthalpy (because the energy is being released in the process) in their respective periods of the periodic table. Was this answer helpful?
Which has more negative electron affinity Si or p?
The extra repulsion involved leads to the electron affinity being lower for P than for Si despite the higher nuclear charge.
How do you determine electron affinity?
1:245:43Electron Affinity - Chemistry Tutorial - YouTubeYouTubeStart of suggested clipEnd of suggested clipEven though we said that usually we see electron affinity values become more negative as we go fromMoreEven though we said that usually we see electron affinity values become more negative as we go from left to right across the periodic table.
Which of the following has highest EA a F (-) B Cl (-) C Li (+) d Na (+)?
F− ions has the highest electron affinity (EA).
Which period has the highest electron affinity?
Hence, Cl has the highest electron affinity. So, option (b)- Period 3, group 17 is the correct answer.
Which has higher electron affinity fluorine or neon?
Thus, noble gases have the least electron affinity in a period. Hence, we can conclude that in a period, fluorine (halogen) has higher electron affinity than neon (noble gas).
Why is the first electron affinity negative?
First electron affinities are typically negative values. This is because when an electron is added to a neutral atom, energy is released in an exothermic process. The second electron affinity is the energy required to add a second electron to an anion.
What is a negative affinity chemistry?
The electron affinity is the potential energy change of the atom when an electron is added to a neutral gaseous atom to form a negative ion. So the more negative the electron affinity the more favourable the electron addition process is.
Why is electron affinity of fluorine negative?
Electron affinity is the energy released when 1 mole of gaseous atoms each acquire an electron to form 1 mole of gaseous 1- ions. Fluorine has a negative value as energy is released when the electron is gained. This is because there is an attraction between the protons in the nucleus and the added electrons.
Which has higher electron affinity lithium or beryllium?
Lithium has an incompletely filled 2s subshell while beryllium has a filled subshell. Therefore, lithium has affinity to receive electrons in 2s sub-shell but for beryllium, a still higher energy 2p level is needed. Hence beryllium resists gaining extra electrons in higher energy 2p orbital.
Which has more electron affinity O or O?
Actually, since O− is negatively-charged, it would be more difficult to accept an electron, and significant coulombic repulsion would make the electron affinity of O− more positive than that of O (which is negative).
Why does Mercury have the lowest electron affinity?
Mercury has lowest Electron Affinity. The reason is generally metals have lower electron affinity , in those metals Mercury attracts electrons most weakly hence it has lowest Electron Affinity.
Why do halogens have the highest electron affinity?
The halogens, which belong to Group VIIA, have the highest electron affinities because adding an electron to an atom results in a totally filled shell. Hence halogens have the greatest electron affinity.
Which of these group 16 elements has the highest electron affinity?
According to electron gain enthalpy, oxygen has the highest electron affinity while selenium has the lowest.
Why fluorine has more electron affinity than chlorine?
the electron affinity of the fluorine is less than chlorine because the size of fluorine is too small as size decreases from left to right inside period, whereas chlorine has a larger size to accommodate electrons hence electron affinity of chlorine is more than fluorine.
Which element has the highest most negative electron affinity group of answer choices S Cr Kr Mg Li?
Given the remaining choices, the element with the highest electron affinity is thus sulfur (S).
Which element has the highest first electron affinity quizlet?
Helium has the highest first ionization energy and is the element that requires the most energy to remove one electron.
Which group has the highest electron affinity within a period?
Electron affinity is the energy released when an electron is added to a gaseous atom or ion. Electron affinity generally decreases going down a group and increases left to right across a period. Nonmetals tend to have the highest electron affinities.
When comparing the two elements GA and BR the element with the more negative electron affinity is?
bromineAnswer and Explanation: Gallium and bromine belong to the same period but gallium is a Group 3A element while bromine is a Group 7A element. This means that bromine has a smaller atomic radius and greater nuclear charge. Hence, bromine will have a higher electron affinity (more negative than gallium).
What does halogens indicate?
Hence they indicate that more energy is released when an electron is added to the neutral atom. You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
What is the energy change of an electron?
In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. And this amount of energy change (ΔE) is called electron affinity. This energy change (ΔE) can be positive, negative or zero. And the sign of Electron Affinity (E EA) is opposite to the sign of energy change (ΔE).
What is electron affinity?
Electron affinity is the amount of energy change (ΔE) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. ...
Is the Interactive Periodic Table free?
Checkout Interactive Periodic table and download it’s high resolution image now ( It’s FREE)
Is the sign of electron affinity positive or negative?
Hence the sign of Electron Affinity (EEA) will be positive. For endothermic reaction (i.e when energy is absorbed), the change in energy (ΔE) is positive. Hence the sign of Electron Affinity (EEA) will be negative. For neutral process (i.e when energy is neither absorbed nor released), the value of Electron Affinity (E EA) will be zero.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.