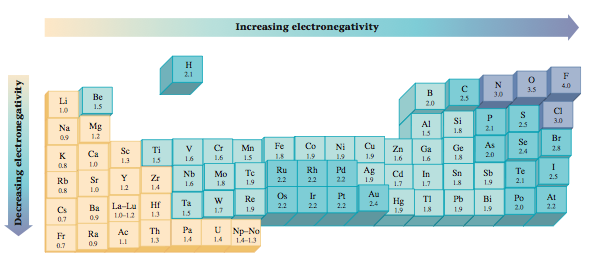
Which element is the least electronegative?
Thus, fluorine is the most electronegative of the elements (ignoring noble gases) and caesium is the least electronegative.
What element has largest electronegativity?
Therefore, fluorine has the highest electronegativity out of all of the elements. Because fluorine has seven valence electrons, it only needs one more electron to acheive a noble gas configuration (eight valence electrons).
What makes an atom more electronegative?
Electronegativity, symbolized as χ, is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity, the more an atom or a ...
Where is the highest electronegativity found?
Where is the highest electronegativity found? Electronegativity increases from bottom to top in groups, and increases from left to right across periods. Thus, fluorine is the most electronegative element, while francium is one of the least electronegative. 37 Related Question Answers Found
What is the first element in the periodic table?
The first chemical element is Actinium and the last element is Fluorine.
How many elements are in chemistry?
This list contains the 118 elements of chemistry.
Which element has the lowest electronegativity?
The element with the lowest electronegativity value is francium, which has an electronegativity of 0.7. This value uses the Pauling scale to measure electronegativity.
What is the difference between electronegativity and electronegativity?
Anne Marie Helmenstine, Ph.D. Updated February 13, 2018. Electronegativity is a measure of an atom's ability to attract electrons to form a chemical bond. High electronegativity reflects a high capacity to bond electrons, while low electronegativity indicates low ability to attract electrons.
What is the name of the scientist who studies the ability of an atom to attract electrons to form a chemical?
Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels. Electronegativity is a measure of an atom's ability to attract electrons to form a chemical bond.
Is electronegativity a property?
Electronegativity is not a uniquely defined property and may depend on the definition . The suggested values are all taken from WebElements as a consistent set.
Is francium more electronegative than caesium?
However, francium is expected and, to a small extent, observed to be more electronegative than caesium. See francium for details.
What Is Electronegativity?
Electronegativity values are used for two main reasons. The first reason to examine the bond between different atoms to predict whether the bond will be covalent (molecular) or ionic. The electron pairs that result in a covalent bond are also known as bonding pairs or shared pairs. There are many kinds of interactions involving covalent or molecular bonds.
What is the name of the pair of electrons that form a covalent bond?
The electron pairs that result in a covalent bond are also known as bonding pairs or shared pairs. There are many kinds of interactions involving covalent or molecular bonds. But when you pair up ions that are oppositely charged attracted electrostatically, then you would get what is known as an ionic compound.
What is a nonpolar bond?
A nonpolar bond is the result of a zero difference in electronegativity. To establish the polarity of a bond, you would need to check out the difference in electronegativity between the atoms involved.
What is the key piece of information in the periodic table?
A key piece of information they contain is the electronegativity value of each of the elements.
Is an anions negatively charged?
While anions are negatively charged, cations are positively charged. The electron can no longer be conceived as a single, small granule of electricity; it must be associated with a wave, and his wave is no myth; its wavelength can be measured and its interferences predicted. – Louise de Broglie.