Lithium, Hydrogen, Sodium, or Oxygen, Sulfur, Selenium They are all in the same column. Find three elements in the same family and list them. 4. Oxygen, Carbon, Nitrogen, Aluminum, Silicon, Phosphorous They are all in the same row.
Full Answer
What do elements in an element family have similar?
Oxygen Group or Chalcogens Family of Elements
- Group 16 or VIA
- Oxygen Group or Chalcogens
- 6 valence electrons
- Diverse properties, changing from nonmetallic to metallic as you move down the family
- Best-known member: oxygen
Why do elements in the same family have similar properties?
Mendeleev predictions include:
- Eka-boron (scandium)
- Eka-aluminium (gallium)
- Eka-manganese (technetium)
- Eka-silicon (germanium)
What do elements in each family have in common?
family characteristics and properties. Elements of the same family show similar characteristics such as chemical re-activity or the same number of valence electrons.
What is true about elements in a family?
Elements are classified into families because the three main categories of elements (metals, nonmetals, and semimetals) are very broad. The characteristics of the elements in these families are determined primarily by the number of electrons in the outer energy shell.
What elements are in the same family group?
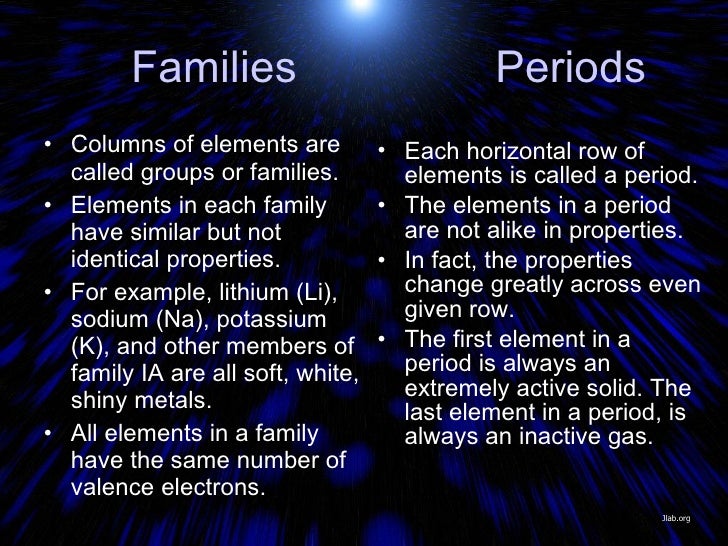
Summary. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior. All the members of a family of elements have the same number of valence electrons and similar chemical properties.
What are 3 elements in the same family?
Lithium, Hydrogen, Sodium, or Oxygen, Sulfur, Selenium They are all in the same column. Find three elements in the same family and list them. 4. Oxygen, Carbon, Nitrogen, Aluminum, Silicon, Phosphorous They are all in the same row.
What does elements in the same family mean?
Element families are elements that have the same number of valence electrons. Most element families are a single column of the periodic table, although the transition elements consist of several columns, plus the elements located below the main body of the table.
How are elements in the same family alike?
The elements in the same group have a similar number of valence electron. They have an identical number of electrons in their outermost shell. e.g. All the alkali metals in Group 1 have 1 valence electron, so they all tend to react the same way with other substances.
What are Group 3 elements called?
Group 3 is the first group of transition metals in the periodic table.
What is a compound with 3 elements called?
Here is a video which summarizes how to name ternary (3+ elements) ionic compounds.
Why are elements in the same group?
Elements in the group have similar chemical properties. This is because their atoms have the same number of electron in the highest occupied energy level.
Which two elements are in the same period?
The first period contains fewer elements than any other, with only two, hydrogen and helium.
How many element families are there?
nine element families9 Element Families Another common method of categorization recognizes nine element families: Alkali Metals: Group 1 (IA) - 1 valence electron.
What are the 7 families of the periodic table?
These families are alkali metals, alkaline earth metals, transition metals, post-transition metals, metalloids, halogens, noble metals, and noble gases. Many of these families belong to a single group on the periodic table. However, not all of the families overlap with periodic table groups.
Why do Group 7 elements have similar properties?
Atoms of group 7 elements all have seven electrons in their outer shell. This means that the halogens all have similar chemical properties.
Why elements in the same family have similar physical and chemical properties?
So, we can say that elements in a group have the same physical and chemical properties because their atoms have the same number of valence electrons or same valence shell electronic configuration.
What are the 3 elements of life?
Many elements make up the human body, but only three occur in abundance. These elements, oxygen, carbon, and hydrogen, combine to form the constituents of some of the most essential processes in the human body, such as cellular respiration.
What is a family of elements called?
An element family is also called an element group. Because of the potential for confusion, the IUPAC prefers element groups be labelled by number rather than name.
What element is in group 3 and 3rd period?
aluminumGroup 3A (or IIIA) of the periodic table includes the metalloid boron (B), as well as the metals aluminum (Al), gallium (Ga), indium (In), and thallium (Tl). Boron forms mostly covalent bonds, while the other elements in Group 3A form mostly ionic bonds....Group 3A.4ASi5AP6AS7ACl8AAr12 more columns
What is an element family?
Element families are elements that have the same number of valence electrons. Most element families are a single column of the periodic table, although the transition elements consist of several columns, plus the elements located below the main body of the table. An example of an element family is the nitrogen group or pnictogens.
What are element families and element groups?
For the most part, element families and element groups are the same things. Both describe elements that share common properties, usually based on the number of valence electrons. Usually, either family or group refers to one or more columns of the periodic table.
How many valence electrons does a halogen have?
The halogens have 7 valence electrons, while the noble gasses have 8 valence electrons (or 0, depending on how you look at it).
Do element groups always have the same number of valence electrons?
Element groups, defined this way, do not always have the same number of valence electrons. For example, the halogens and noble gasses are distinct element groups, yet they also belong to the larger group of nonmetals.
Which elements have similar physical properties?
For example, Group 1 elements (lithium, sodium, potassium, rubidium, cesium and francium) have similar physical properties like, metallic luster, softness , etc. Also they have similar chemical properties. For example, all the alkali metals (group 1 elements) are highly reactive to water. Thus all the elements of group 1 have the same number ...
Which group of elements has the same number of valence electrons?
Elements of group 17 are highly electronegative and have higher ionisation enthalpy. They react with water and form halides. Thus all the elements of group 17 have the same number of valence electrons (i.e 7) and they show common properties.
What are the vertical columns in the periodic table called?
Modern periodic table consist of groups and periods. The vertical columns are called groups. The elements which are in the same groups have the same number of valence electrons (i.e same number of electrons in the outermost shell). Also they possess almost similar properties.
How many valence electrons does each element have?
All the elements of 1st group (H, Li, Na, K, Rb, Cs, Fr) have one valence electron.
How many electrons are in the 13th group?
All the elements of 13th group have 3 electrons in its outermost orbit.
How many electrons are in the outer shell of a 14th group?
14th group elements have 4 electrons in outer shell, not 14.
Which electrons participate in chemical reactions?
Thus electrons of both s-orbitals as well as d-orbitals participate in chemical reaction.