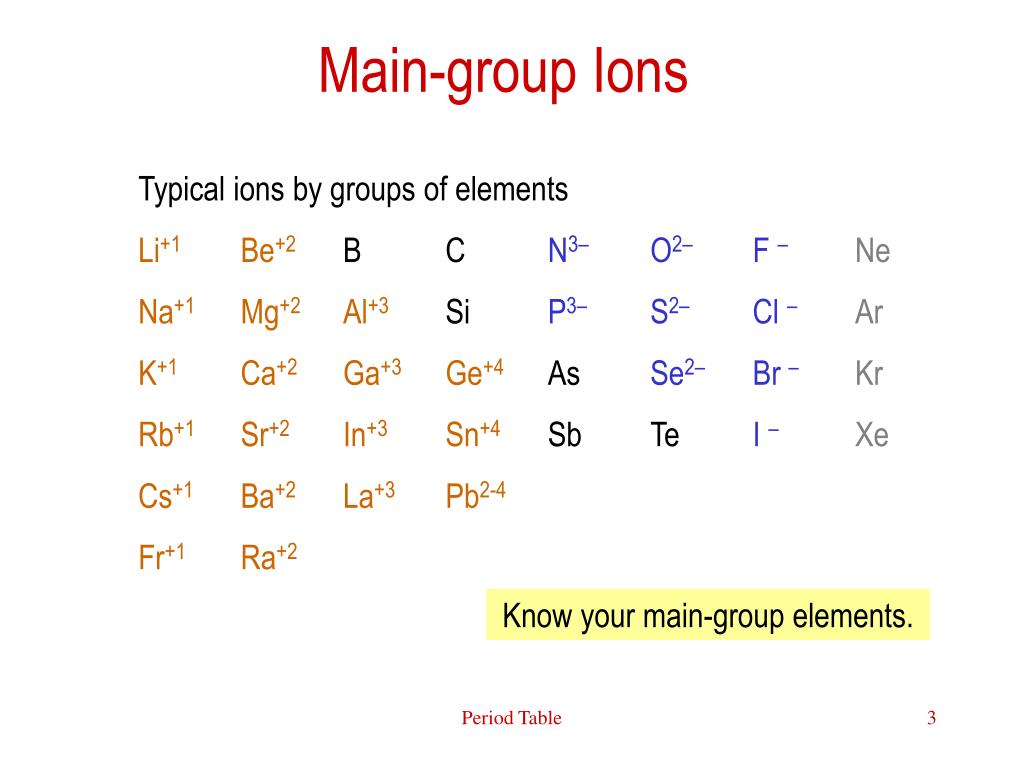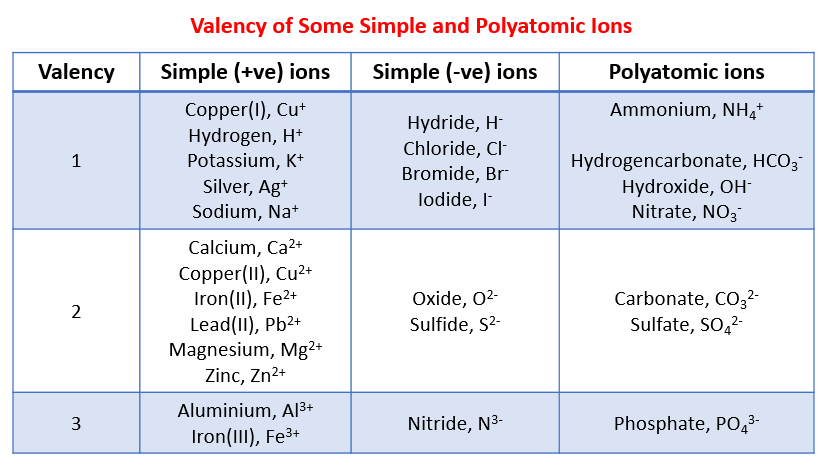
| Number | Element | Charge |
| 1 | Hydrogen | 1+ |
| 2 | Helium | 0 |
| 3 | Lithium | 1+ |
| 4 | Beryllium | 2+ |
Full Answer
What is the difference between ions and elements?
Because it only has one type of apple, it would be an element. An ion is a non-neutrally charged atom. A neutral atom has the same amount of protons and electrons which is why it is neutral. But in a non-neutral atom, there is a lack (Cation, +) or excess of electrons (Anion, -).
Which elements would form an ion?
Ionic compounds generally form between elements that are metals and elements that are nonmetals. For example, the metal calcium (Ca) and the nonmetal chlorine (Cl) form the ionic compound calcium chloride (CaCl2). Read, more elaboration about it is given here.
What are four examples of elements?
- Elements found on Earth and Mars are exactly the same.
- Hydrogen is the most common element found in the universe. ...
- Isotopes are atoms of the same element, with different numbers of neutrons.
- In ancient times the elements referred to fire, earth, water, and air.
- Helium is the second most common element in the universe, but is very rare on the Earth.
Which elements have fewest protons?
Which element has the fewest protons and is considered the lightest which element is the heaviest? Hydrogen. Hydrogen is the lightest and most common element in the universe, consisting of just a single proton and a single electron, with the chemical symbol H.

What element is ions?
An ion is any element that contains a different number of protons and electrons resulting in either a positively or negatively charged atom.
How do you know if an element is an ion?
When an atom is attracted to another atom because it has an unequal number of electrons and protons, the atom is called an ION. If the atom has more electrons than protons, it is a negative ion, or ANION. If it has more protons than electrons,it is a positive ion.
What elements are not ions?
Elements in group 18 of the periodic table – the “noble gases”, tend not to form ions due to the arrangement of their electrons which makes them generally unreactive.
What are examples of ions?
Common examples include sodium, potassium, calcium, chloride, and bicarbonate. These substances are known as electrolytes. Ions can be created using radiation such as x-rays.
Is oxygen an ion?
As the oxygen molecule captured an electron, the oxygen molecule is defined as negative oxygen ion (O2−)4,5. The negative oxygen ions are also known as “air vitamin” due to its important meanings to human life activities.
Is carbon an ion?
In addition, the ionization energy of the atom is too large and the electron affinity of the atom is too small for ionic bonding to occur. For example: carbon does not form ionic bonds because it has 4 valence electrons, half of an octet. To form ionic bonds, Carbon molecules must either gain or lose 4 electrons.
How many ions are there?
There are two types of ions : cations. anions.
Is an electron an ion?
Ions vs Electrons There are many differences between electrons and ions; size, charge, and nature are some of them. Electrons are negatively charged micro particles and ions are either negatively or positively charged molecules or atoms.
What is ions in simple words?
1 : an atom or group of atoms that carries a positive or negative electric charge as a result of having lost or gained one or more electrons. 2 : a charged subatomic particle (such as a free electron) Ion.
Is Salt an ion?
In chemical terms, salts are ionic compounds. To most people, salt refers to table salt, which is sodium chloride. Sodium chloride forms from the ionic bonding of sodium ions and chloride ions. There is one sodium cation (Na+) for every chloride anion (Cl–), so the chemical formula is NaCl (Fig.
What are called ions?
ion, any atom or group of atoms that bears one or more positive or negative electrical charges. Positively charged ions are called cations; negatively charged ions, anions.
Is h2o an ion?
Likewise, a water molecule is ionic in nature, but the bond is called covalent, with two hydrogen atoms both situating themselves with their positive charge on one side of the oxygen atom, which has a negative charge.
How do you know if an element is ionic or covalent?
If a compound is made from a metal and a non-metal, its bonding will be ionic. If a compound is made from two non-metals, its bonding will be covalent.
How can you tell the charge of an ion?
0:533:12How to Identify the Charge of an Ion : Chemistry Lessons - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo if it's an ion it has eight already which means that it has one more electron than it has aMoreSo if it's an ion it has eight already which means that it has one more electron than it has a neutral charge. So a chloride a chlorine ion or chloride is going to have a charge of negative one.
What is the difference between an ion and an element?
Atoms where the electrons and protons are not equal are called ions. Ions are charged particles. They can be either positively charged ions or negatively charged ions....Difference between Atom and IonATOMIONThe Smallest unit of elementSingle particle or collection of particles4 more rows
How do you know if an element is a cation or anion?
Cations are positively-charged ions (atoms or groups of atoms that have more protons than electrons due to having lost one or more electrons). Anions are negatively-charged ions (meaning they have more electrons than protons due to having gained one or more electrons).
How many types of ions are there?
There are only two types of ions- Cations and anions. Cations are positively charged particles or ions and anions are negatively charged particles...
How do you determine ionic charge?
There is a quick way to calculate the charge on an ion: the number of charges on an ion formed by a metal is equal to the metal’s group number. The...
How do you read an ion?
When writing an ion symbol, the one- or two-letter element symbol comes first, followed by a superscript. The superscript indicates the number of c...
Which group in the periodic table cannot form ions?
Since the Group 8A elements have a full octet of eight valence electrons in their highest-energy orbitals, they have a low tendency to gain or lose...
Can ions exist on their own?
Ions of like charge repel each other, and ions of opposite charge attract each other. Therefore, ions do not usually exist on their own but will bi...
What is an ion?
An ion is defined as an atom or molecule that has gained or lost one or more of its valence electrons, giving it a net positive or negative electrical charge. In other words, there is an imbalance in the number of protons (positively charged particles) and electrons ...
What does a number mean in an anions?
A number, if present, precedes the plus sign. If only a "+" is present, it means the charge is +1. For example, Ca 2+ indicates a cation with a +2 charge. Anions are ions that carry a net negative charge. In anions, there are more electrons than protons. The number of neutrons is not a factor in whether an atom, functional group, ...
Why do ions carry a net positive charge?
Cations are ions that carry a net positive charge because the number of protons in the species is greater than the number of electrons. The formula for a cation is indicated by a superscript following the formula that indicates the number of the charge and a "+" sign. A number, if present, precedes the plus sign.
Why are cations and anions attracted to each other?
Because they carry opposite electrical charges, cations and anions are attracted to each other. Cations repel other cations; anions repel other anions. Because of the attractions and repulsion between ions, they are reactive chemical species.
What is a monatomic ion?
If an ion consists of a single atom, it is called a monatomic ion. An example is the hydrogen ion, H +. By contrast, polyatomic ions, also called molecular ions, consist of two or more atoms. An example of a polyatomic ion is the dichromate anion:
Where did the word "ion" come from?
The term "ion" was introduced by English chemist and physicist Michael Faraday in 1834 to describe the chemical species that travels from one electrode to another in aqueous solution. The word ion comes from the Greek word ion or ienai, which means "to go.".
Is the number of neutrons a factor?
The number of neutrons is not a factor in whether an atom, functional group, or molecule is an anion. Like cations, the charge on an anion is indicated using a superscript after a chemical formula. For example, Cl - is the symbol for the chlorine anion, which carries a single negative charge (-1). If a number is used in ...