What family does lanthanum belong to?
Lanthanum itself is sometimes considered to be a d-block element, because it has no electrons in an f orbital, but it does have one electron in a d orbital. It has also been argued that, because the word lanthanide literally means “like lanthanum”, lanthanum itself cannot be a lanthanide.
What is the most common use for lanthanum?
Lanthanum
- History and Discovery. Lanthanum was discovered in the 19th century, this came precisely in 1838 by a Swedish chemist known as Carl Mustav Gosander at the Karolinska Institute in Stockholm.
- Lanthanum
- Occurrence. Lanthanum is not a rare metal. ...
- Physical Characteristics. ...
- Chemical Characteristics. ...
- Significance and Uses. ...
- Health Effects. ...
- Isotopes of Lanthanum. ...
What is lanthanum named after?
The name lanthanum was given to the newly discovered metal, which was derived from the Greek word lanthanein, that means hidden. The symbol of lanthanum is La. Lanthanum is not a rare metal. It is ranked as the twenty-eighth most abundant metal in the earth’s crust and is thrice (39 mg/kg of the earth’s crust) as abundant as lead.
What is the normal phase of lanthanum?
The transition of a substance directly from the solid to the gas phase without passing through a liquid phase. Density is the mass of a substance that would fill 1 cm 3 at room temperature.
See more

What group do the lanthanides belong to?
3B Group of Elements The third row of the 3B group contains all of the elements between element 57 (lanthanum) and element 71 (lutetium). These elements are grouped together and called the lanthanides.
Which element is in the lanthanide family?
The elements cerium (Ce, atomic no. 58) through lutetium (Lu, atomic no. 71) are commonly known as the lanthanide series. Remark: This description of 'the lanthanides' is based on the IUPAC (International Union of Pure and Applied Chemistry) definition.
What does lanthanum belong to?
rare earth elementsLanthanum is traditionally counted among the rare earth elements.
What family are the lanthanides and actinides a part of?
The lanthanides and actinides are groups of elements in the periodic table. They are the elements that are often listed below the main section of the periodic table. There are thirty total elements in the lanthanides and actinides. They are often called the "inner transition metals."
Are lanthanides metalloids?
The metalloids are B, Si, Ge, As, Sb, Te, and Po. They sometimes behave as semiconductors (B, Si, Ge) rather than as conductors. Lanthanides. The lanthanides comprise elements 57 (lanthanum, hence the name of the set) through 71.
What is the family name for Group 17?
halogenhalogen, any of the six nonmetallic elements that constitute Group 17 (Group VIIa) of the periodic table. The halogen elements are fluorine (F), chlorine (Cl), bromine (Br), iodine (I), astatine (At), and tennessine (Ts).
What group and period is lanthanum?
Fact boxGroupLanthanidesMelting pointPeriod6Boiling pointBlockdDensity (g cm−3)Atomic number57Relative atomic massState at 20°CSolidKey isotopes2 more rows
What element group does argon belong to?
Group 18argon (Ar), chemical element, inert gas of Group 18 (noble gases) of the periodic table, terrestrially the most abundant and industrially the most frequently used of the noble gases.
Is LA a lanthanide?
Lanthanum is a chemical element with symbol La and atomic number 57. Classified as a lanthanide, Lanthanum is a solid at room temperature.
Which reactive family is found in Group 2?
alkaline-earth metalalkaline-earth metal, any of the six chemical elements that comprise Group 2 (IIa) of the periodic table. The elements are beryllium (Be), magnesium (Mg), calcium (Ca), strontium (Sr), barium (Ba), and radium (Ra).
Are lanthanide series metals?
The lanthanides are a series of 14 metallic elements that appear at the bottom of the periodic table. Lanthanum, the element preceding the lanthanides in the periodic table, is usually also included in a discussion of the lanthanides since all 15 elements have very similar properties.
Why are the actinides a family?
The lanthanides (or rare-earth elements) and actinides are two families that are related because they both result from electrons being added into an f sub-level.
Overview
History
In 1751, the Swedish mineralogist Axel Fredrik Cronstedt discovered a heavy mineral from the mine at Bastnäs, later named cerite. Thirty years later, the fifteen-year-old Wilhelm Hisinger, from the family owning the mine, sent a sample of it to Carl Scheele, who did not find any new elements within. In 1803, after Hisinger had become an ironmaster, he returned to the mineral with Jöns …
Characteristics
Lanthanum is the first element and prototype of the lanthanide series. In the periodic table, it appears to the right of the alkaline earth metal barium and to the left of the lanthanide cerium. Its placement has been disputed, but most who study the matter along with a 2021 IUPAC provisional report consider lanthanum to be best placed as the first of the f-block elements. The 57 electr…
Compounds
Lanthanum oxide is a white solid that can be prepared by direct reaction of its constituent elements. Due to the large size of the La ion, La2O3 adopts a hexagonal 7-coordinate structure that changes to the 6-coordinate structure of scandium oxide (Sc2O3) and yttrium oxide (Y2O3) at high temperature. When it reacts with water, lanthanum hydroxide is formed: a lot of heat is evolved in the reaction and a hissing sound is heard. Lanthanum hydroxide will react with atmos…
Occurrence and production
Lanthanum is the third-most abundant of all the lanthanides, making up 39 mg/kg of the Earth's crust, behind neodymium at 41.5 mg/kg and cerium at 66.5 mg/kg. It is almost three times as abundant as lead in the Earth's crust. Despite being among the so-called "rare earth metals", lanthanum is thus not rare at all, but it is historically so named because it is rarer than "common earths" such a…
Applications
The first historical application of lanthanum was in gas lantern mantles. Carl Auer von Welsbach used a mixture of lanthanum oxide and zirconium oxide, which he called Actinophor and patented in 1886. The original mantles gave a green-tinted light and were not very successful, and his first company, which established a factory in Atzgersdorf in 1887, failed in 1889.
Biological role
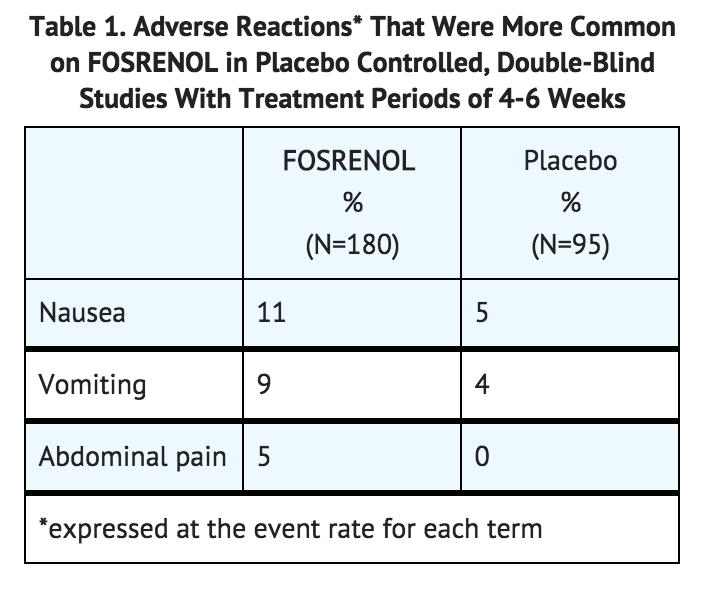
Lanthanum has no known biological role in humans. The element is very poorly absorbed after oral administration and when injected its elimination is very slow. Lanthanum carbonate (Fosrenol) was approved as a phosphate binder to absorb excess phosphate in cases of end stage renal disease.
While lanthanum has pharmacological effects on several receptors and ion channels, its specifi…
Precautions
Lanthanum has a low to moderate level of toxicity and should be handled with care. The injection of lanthanum solutions produces hyperglycemia, low blood pressure, degeneration of the spleen and hepatic alterations. The application in carbon arc light led to the exposure of people to rare earth element oxides and fluorides, which sometimes led to pneumoconiosis. As the La ion is similar in size to the Ca ion, it is sometimes used as an easily traced substitute for the latter in …