
There are many functional groups in both small and large biomolecules that act as acids and bases. Common weak acids are carboxylic acids and derivatives of phosphoric acid which become negatively charge on donation of a proton. Common weak base are amines which become positively charged on protonation.
Full Answer
What are acidic functional groups?
Acidic functional groupsare those that can donate (or lose) a proton (H+). From a very simplistic view, an acid can be represented as: Page 1of 32 UnionizedI onized pK Un UnionizedIonized There are two key features of an acidic functional group: the presence of a hydrogen atom that can dissociate from the group (H+), and the ability of the
What are the functional groups in biological molecules?
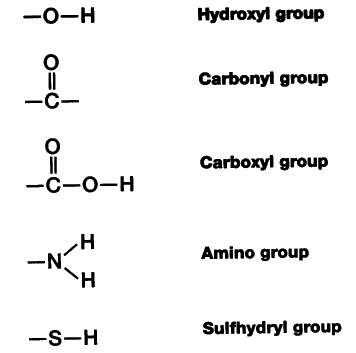
A few of the most important functional groups in biological molecules are shown in the table below. Functional groups can be classified as hydrophobic or hydrophilic based on their charge and polarity characteristics. The only hydrophobic group below is the methyl (CH) group, which is nonpolar.
Are amino acids acidic or basic at pH?
Since carboxyl groups can release H into solution, they are considered acidic. Charged (forms R-NH) at the pH of most biological systems. Since amino groups can remove H from solution, they are considered basic. Charged (forms R-OPO) at the pH of most biological systems.
What are the functional groups found in amines?
phosphonates, and sulfates. • Identify the following basic functional groups: aliphatic amines, alicyclic amines (aka saturated heterocycles), aromatic amines, imines, hydrazines, amidines, guanidines, and nitrogen containing aromatic heterocycles.
Which functional groups are bases?
Since amino groups can remove H +start superscript, plus, end superscript from solution, they are considered basic.
Which of functional groups behaves as an acid?
Which of these functional groups behaves as an acid? By donating hydrogen ions, carboxyl groups act as an acid.
Are hydroxyl groups acidic or basic?
acidicThe hydroxyl group is an OH group that is attached to any of the molecules which possess an acidic property as it has an ability to lose the hydrogen atom. The hydroxyl group which is present at the 7th position in the molecules is likely to be most acidic in nature.
Which functional groups are most acidic?
Carboxylic Acid The carboxylate is resonance stabilised to a very significant extent, as the negative charge on oxygen is delocalised extensively between 2 electronegative oxygens. This makes the carboxylate very stable, hence carboxylic acid is the most acidic functional group.
Are alcohol functional groups acidic or basic?
Therefore, alcohols are more acidic than terminal alkynes, amines, and alkanes but less acidic than hydrogen halides. Typical alcohols like methanol or ethanol are almost as acidic and basic as water. However, phenols are more acidic than alcohols but less acidic than carboxylic acids.
Which of these functional groups behaves as an acid quizlet?
Which functional group behaves as an acid? Carboxyl group; By donating hydrogen ions, carboxyl groups act as an acid.
How do you know if a functional group is acidic or basic?
Since amino groups can remove H+ from solution, they are considered basic. Charged, ionizes to release H+. Since phosphate groups can release H+ ions into solution, they are considered acidic.
Are aldehydes acidic or basic?
weak acidicAldehydes are weak acidic in nature due to the presence of alpha hydrogen atom in it. The –CHO functional group is present in aldehydes containing carbonyl group and a hydrogen atom.
Is amine acidic or basic?
basicAmine are basic and easily react with the hydrogen of acids which are electron poor as seen below. Amines are one of the only neutral functional groups which are considered basis which is a consequence of the presence of the lone pair electrons on the nitrogen.
Is carboxyl group acidic or basic?
Carboxylic acids, containing a carboxyl group (-COOH), are the most acidic class of standard organic molecules.
Is COOH acid or base?
Carboxylic acid is an organic compound containing a carboxyl group (COOH) attached to an alkyl or aryl group. They react with metals and alkalis to generate carboxylate ions. These reactions of carboxylic acids indicate their acidic nature.
Is carbonyl group acidic or basic?
Normally the Carboxyl groups are acidic in nature. The Carbonyl group is also uncharged but is a little hydrophillic.
Which functional group acts most like an acid in water?
Which of the functional groups below acts most like an acid in water? carboxyl.
How do you know if a functional group is acidic or basic?
Since amino groups can remove H+ from solution, they are considered basic. Charged, ionizes to release H+. Since phosphate groups can release H+ ions into solution, they are considered acidic.
Does organic phosphate act as an acid?
The forms phosphoric acid, dihydrogen phosphate ion, hydrogen phosphate ion, and phosphate ion all act and behave as individual weak acids.
What is the functional group of carboxylic acid?
The carboxyl functional group that characterizes the carboxylic acids is unusual in that it is composed of two functional groups: (1) the carboxyl group and (2) of a hydroxyl group bonded to a carbonyl group. It is often written in condensed form as –CO2H or –COOH.
What are functional groups?
Functional groups. Large biological molecules are generally composed of a carbon skeleton (made up of carbon and hydrogen atoms) and some other atoms, including oxygen, nitrogen, or sulfur. Often, these additional atoms appear in the context of functional groups. Functional groups are chemical motifs, or patterns of atoms, ...
Which group is hydrophilic?
One example of a strongly hydrophilic group is the carboxyl group (COOH), which can act as an acid and lose a proton to form a negatively-charged carboxylate ion (COO ). Carboxyl groups are commonly found in amino acids, fatty acids, and other biomolecules. An example of a less hydrophilic group is the carbonyl group (C=O), an uncharged but polar (contains partial positive and partial negative charges) functional group. Carbonyls are found in many different biological molecules, including proteins, peptides, and carbohydrates.
What are some examples of less hydrophilic groups?
An example of a less hydrophilic group is the carbonyl group (C=O), an uncharged but polar (contains partial positive and partial negative charges) functional group. Carbonyls are found in many different biological molecules, including proteins, peptides, and carbohydrates.
Why are short molecules soluble in water?
Direct link to Matt B's post “The short molecules are s...”. more. The short molecules are soluble in polar substances like water because they are relatively polar. The longer the carbon chain is however, the greater the non-polar tail is, and the less soluble aldehydes and ketones become.
What are the two types of fuels that are made up of carbon and hydrogen?
Hydrocarbons, made up entirely of carbon and hydrogen atoms, make wonderful combustion fuels (such fuels include propane, butane, and the bulk of commercial gasoline).
Why are alkanes less reactive than alkenes?
Alkanes are less reactive than alkenes. This is because alkenes have double bonds (C=C). It is easy to break just one of these bonds in the double bond and make a reaction happen, but breaking the C-C single bond in an alkane is difficult. Comment on Nahin Khan's post “Alkanes are less reactive...”.
Is a charged group positive or negative?
more. A charged group is either positive or negative (gains or loses an electron) and a polar group contains atoms that have a difference in electronegativity. Comment on Citrus's post “A charged group is either...”. Button opens signup modal.
What is a functional group?from askinglot.com
A functional group is a group of atoms and bonds within a molecule. The functional group helps to determine whether something is acid, low pH, or basic and has a high pH. An example of an acidic functional group is a carboxyl. The carboxyl functional group is acidic because it is a proton (H+) donator when in solution.
Which class of organic compounds contains a carboxyl functional group?from askinglot.com
carboxylic acid : Any of a class of organic compounds containing a carboxyl functional group—a carbon with one double bond to an oxygen and a single bond to another oxygen, which is in turn bonded to a hydrogen. alcohol: Class of organic compounds containing a hydroxyl functional group.