
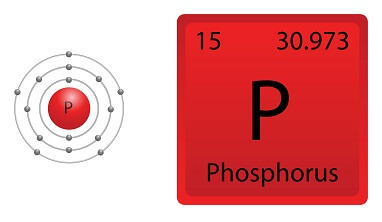
Phosphorus is in Period 3and Group 15 of the periodic table. It has 15 protons, so it has the atomic number 15. This also means it is the 15th element on the periodic table. The chemical symbol for phosphorus is P.
| Group | 15 | Melting point |
|---|---|---|
| Period | 3 | Boiling point |
| Block | p | Density (g cm−3) |
| Atomic number | 15 | Relative atomic mass |
| State at 20°C | Solid | Key isotopes |
What is group and period does phosphorus belong to?
Phosphorus (P) is a chemical element of the periodic table, located in the group 15 and the period 3, and is having the atomic number 15. It is a soft, waxy, yellow-white reactive nonmetal, whose name comes from the Greek word “phosphoros”, which means light bringer or light carrier.It is a member of the pnictogen group and is the 11 th most abundant element on earth.
What are unusual facts about phosphorus?
Unusual Facts About Phosphorus White phosphorus is usually stored underwater as it ignites in moist air at about 30 degrees centigrade. Most of the phosphorus on Earth's crust comes from phosphate rock that is mined all over the world. More than 50 million tonnes of phosphorus are made every year, and it has multiple uses.
What are facts about phosphorus?
Facts About Phosphorus. Phosphorus is in Period 3 and Group 15 of the periodic table. It has 15 protons, so it has the atomic number 15. This also means it is the 15th element on the periodic table.
What is Phosphorus mainly used for?
Phosphorus is used to treat anxiety, fears, dandruff, cough, pneumonia, nose bleeding, gastritis, glaucoma, hoarse voice and excessive menstrual bleeding.
See more
Is phosphorus in the 3rd period?
The third period contains eight elements: sodium, magnesium, aluminium, silicon, phosphorus, sulfur, chlorine, and argon.
What is phosphorus in periodic table?
phosphorus (P), nonmetallic chemical element of the nitrogen family (Group 15 [Va] of the periodic table) that at room temperature is a colourless, semitransparent, soft, waxy solid that glows in the dark. phosphorus.
What element is in group 16 period 6?
Group 16 Elements Of Modern Periodic TablePeriodElementAtomic Number3Sulphur164Selenium345Tellurium526Polonium841 more row
In which group does phosphorus P belong to *?
Group 5AGroup 5A (or VA) of the periodic table are the pnictogens: the nonmetals nitrogen (N), and phosphorus (P), the metalloids arsenic (As) and antimony (Sb), and the metal bismuth (Bi).
Is phosphorus a metal or a nonmetal?
non-metalPhosphorus is a non-metal that sits just below nitrogen in group 15 of the periodic table. This element exists in several forms, of which white and red are the best known.
Why is phosphorus a nonmetal?
The phosphorus generally shows +3 and -3 as its oxidation state as by removing three electrons or by adding three electrons it can achieve the stable electronic configuration. So the answer is reactive. Phosphorus is a very reactive non – metal.
Is phosphate and phosphorus same?
The terms phosphate and phosphorus can mean the same thing in test results. So your results may show phosphorus levels rather than phosphate levels. If your test shows you have high phosphate/phosphorus levels, it may mean you have: Kidney disease.
Why is phosphorus called the devil's element?
Some texts refer to phosphorus as the "Devil's Element" because of its eerie glow, tendency to burst into flame, and because it was the 13th known element. Like other nonmetals, pure phosphorus assumes markedly different forms. There are at least five phosphorus allotropes.
How many isotopes of phosphorus are there?
23 isotopes of phosphorus are known, ranging from 25#N#P to 47#N#P. Only 31#N#P is stable and is therefore present at 100% abundance. The half-integer nuclear spin and high abundance of 31 P make phosphorus-31 NMR spectroscopy a very useful analytical tool in studies of phosphorus-containing samples.
Where is phosphorus found?
Phosphorus has a concentration in the Earth's crust of about one gram per kilogram (compare copper at about 0.06 grams). It is not found free in nature, but is widely distributed in many minerals, usually as phosphates. Inorganic phosphate rock, which is partially made of apatite (a group of minerals being, generally, pentacalcium triorthophosphate fluoride (hydroxide)), is today the chief commercial source of this element. According to the US Geological Survey (USGS), about 50 percent of the global phosphorus reserves are in the Arab nations. 85% of Earth's known reserves are in Morocco with smaller deposits in China, Russia, Florida, Idaho, Tennessee, Utah, and elsewhere. Albright and Wilson in the UK and their Niagara Falls plant, for instance, were using phosphate rock in the 1890s and 1900s from Tennessee, Florida, and the Îles du Connétable ( guano island sources of phosphate); by 1950, they were using phosphate rock mainly from Tennessee and North Africa.
How do phosphides form?
Phosphides arise by reaction of metals with red phosphorus. The alkali metals (group 1) and alkaline earth metals can form ionic compounds containing the phosphide ion, P 3−. These compounds react with water to form phosphine. Other phosphides, for example Na 3 P 7, are known for these reactive metals.
Why is phosphorus important for plants?
Artificial phosphate fertilisation is necessary because phosphorus is essential to all living organisms; it is involved in energy transfers, strength of root and stems, photosynthesis, the expansion of plant roots, formation of seeds and flowers, and other important factors effecting overall plant health and genetics.
What is the most common allotrope?
The two most common allotropes are white phosphorus and red phosphorus. The structure of P 4 molecules, determined by gas electron diffraction. From the perspective of applications and chemical literature, the most important form of elemental phosphorus is white phosphorus, often abbreviated as WP.
Why is white phosphorus produced in large facilities?
Production of white phosphorus is conducted in large facilities in part because it is energy intensive. The white phosphorus is transported in molten form.
How many crystals does white phosphorus have?
2 molecules. White phosphorus exists in two crystalline forms: α (alpha) and β (beta). At room temperature, the α-form is stable, which is more common and it has cubic crystal structure and at 195.2 K (−78.0 °C), it transforms into β-form, which has hexagonal crystal structure.
Phosphorus in Periodic table
Phosphorus element is in group 15 and period 3 of the Periodic table. Phosphorus is the p-block element and it belongs to Pnictogens group.
Properties of Phosphorus
The physical and chemical properties of phosphorus element are mentioned below.
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
What is the period number of phosphorus?
In the case of Phosphorus the period number is 3. There is a lot of cool stuff about Phosphorus that people simply don't know. Let me show you...
How is phosphorus obtained?
Found most often in phosphate rock. Pure phosphorus is obtained by heating a mixture of phosphate rock, coke, and silica to about 1450 °C.
How many forms of phosphorus are there?
Like other nonmetals, pure phosphorus assumes markedly different forms. There are at least five phosphorus allotropes. In addition to white phosphorus, there is red, violet, and black phosphorus. Under ordinary conditions, red and white phosphorus are the most common forms.
What color is phosphorus?
Fast Facts: Phosphorus. Appearance: Appearance depends on the allotrope. Phosphorus is a solid at room temperature. It may be white, yellow, red, violet, or black. Discovery: Recognized as an element by Antoine Lavoisier (1777), but officially discovered by Hennig Brand (1669).
How is phosphorus obtained?
Phosphorus is obtained from calcium phosphate by heating the rock in a furnace to yield tetraphosphorus vapor. The vapor is condensed into phosphorus underwater to prevent ignition.
How was phosphorus brought to Earth?
According to a study published in the Proceedings of the National Academies of Sciences, phosphorus may have been brought to Earth by meteorites. The release of phosphorus compounds seen early in Earth's history (yet not today) contributed to the conditions needed for the origin of life. Phosphorus is abundant in the Earth's crust at a concentration of about 1,050 parts per million, by weight.
How much phosphorus is in the human body?
Phosphorus is essential to living organisms. There are about 750 grams of phosphorus in the average adult. In the human body, it's found in DNA, bones, and as an ion used for muscle contraction and nerve conduction. Pure phosphorus, however, can be deadly.
Why is phosphorus called cold fire?
Brand called the new element "cold fire" because it glowed in the dark. The name of the element comes from the Greek word phosphoros, which means "bringer of light.". The form of phosphorus Brand discovered was white phosphorus, which reacts with oxygen in air to produce a green-white light.
What is the primary use of phosphorus?
At least 23 isotopes of the element are known. The primary use of phosphorus is for fertilizer production. The element is also used in flares, safety matches, light-emitting diodes, and steel production. Phosphates are used in some detergents.
Answer
phosphorous have 3 shells ans electronic configuration is 2,8,5 and boron have 2 shells and electronic configuration is 2,3
New questions in Chemistry
The species which is isoelectronic with O2+ is. (a) N2- (b) CN- (c) NO+ (d) N2+ ( explain in detail)
Overview
Characteristics
Phosphorus has several allotropes that exhibit strikingly diverse properties. The two most common allotropes are white phosphorus and red phosphorus.
From the perspective of applications and chemical literature, the most important form of elemental phosphorus is white phosphorus, often abbreviated as WP. It is a soft, waxy solid which consists of tetrahedral P 4 molecules, in w…
Occurrence
In 2013, astronomers detected phosphorus in Cassiopeia A, which confirmed that this element is produced in supernovae as a byproduct of supernova nucleosynthesis. The phosphorus-to-iron ratio in material from the supernova remnant could be up to 100 times higher than in the Milky Way in general.
In 2020, astronomers analysed ALMA and ROSINA data from the massive star-forming region AFG…
Compounds
The most prevalent compounds of phosphorus are derivatives of phosphate (PO4 ), a tetrahedral anion. Phosphate is the conjugate base of phosphoric acid, which is produced on a massive scale for use in fertilisers. Being triprotic, phosphoric acid converts stepwise to three conjugate bases:
H3PO4 + H2O ⇌ H3O + H2PO4 Ka1 = 7.25×10
History
The name Phosphorus in Ancient Greece was the name for the planet Venus and is derived from the Greek words (φῶς = light, φέρω = carry), which roughly translates as light-bringer or light carrier. (In Greek mythology and tradition, Augerinus (Αυγερινός = morning star, still in use today), Hesperus or Hesperinus (΄Εσπερος or Εσπερινός or Αποσπερίτης = evening star, still in use today) and …
Production
In 2017, the USGS estimated 68 billion tons of world reserves, where reserve figures refer to the amount assumed recoverable at current market prices; 0.261 billion tons were mined in 2016. Critical to contemporary agriculture, its annual demand is rising nearly twice as fast as the growth of the human population. The production of phosphorus may have peaked before 2011 and some scient…
Applications
Phosphorus is an essential plant nutrient (the most often limiting nutrient, after nitrogen), and the bulk of all phosphorus production is in concentrated phosphoric acids for agriculture fertilisers, containing as much as 70% to 75% P2O5. That led to large increase in phosphate (PO4 ) production in the second half of the 20th century. Artificial phosphate fertilisation is necessary because phosphorus is essential to all living organisms; it is involved in energy transfers, strengt…
Biological role
Inorganic phosphorus in the form of the phosphate PO 4 is required for all known forms of life. Phosphorus plays a major role in the structural framework of DNA and RNA. Living cells use phosphate to transport cellular energy with adenosine triphosphate (ATP), necessary for every cellular process that uses energy. ATP is also important for phosphorylation, a key regulatory event in cells. Phospholipids are the main structural components of all cellular membranes. Calci…