What is the final result of radioactive decay?
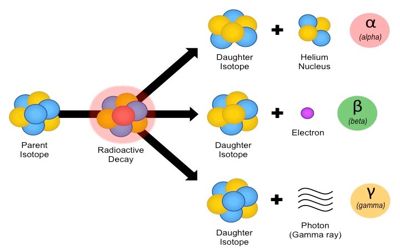
Radioactive decay is a random process by which unstable atoms (with an excess of particles and/or energy) emit radiation to achieve stability. An excess of neutrons and protons can cause this instability, which leads to the emission of alpha particles, beta particles, or high-energy photons (gamma radiation ).
What is radioactive decay and what does it do?
What is radioactive decay and how does it work? Radioactive decay is the spontaneous breakdown of an atomic nucleus resulting in the release of energy and matter from the nucleus. In the process, they will release energy and matter from their nucleus and often transform into a new element. Do all elements decay? All elements with 84 or more ...
What is primarily released in a radioactive decay?
When radioactive atoms decay, they release energy in the form of ionizing radiation (alpha particles, beta particles and/or gamma rays). The energy is called ionizing radiation because it has enough energy to knock tightly bound electrons from an atom's orbit. This causes the atom to become a charged ion.
What can radioactive decay be affected by?
What can radioactive decay be affected by? The half-life of radioactive decay can also be altered by changing the state of the electrons surrounding the nucleus . In a type of radioactive decay called “electron capture”, the nucleus absorbs one of the atom’s electrons and combines it with a proton to make a neutron and a neutrino.

What happens during radioactive decay answers?
Answer and Explanation: During radioactive decay, an unstable nucleus emits subatomic particles to attain stability. It may undergo transmutation to change to some other nucleus or may achieve a stable state by giving off energy in the form of photons.
What is radioactive decay and why does it happen?
Radioactive decay involves the spontaneous transformation of one element into another. The only way that this can happen is by changing the number of protons in the nucleus (an element is defined by its number of protons). There are a number of ways that this can happen and when it does, the atom is forever changed.
Where does radioactive decay happen?
Radioactive decay occurs in atoms that become unbalanced, called radionuclides, according to the U.S. Environmental Protection Agency (opens in new tab). These radionuclides follow their own unique path of decay, transforming into different elements until they reach a stable state.
What is radioactive decay in simple terms?
Radioactive decay is the emission of energy in the form of ionizing radiation. Ionizing radiation can affect the atoms in living things, so it poses a health risk by damaging tissue and DNA in genes..
What is lost during radioactive decay?
When uranium nuclei undergo radioactive decay, some of their mass is converted into kinetic energy (the energy of the moving particles). This conversion of energy is observed as a loss of mass.
What does radioactive decay look like?
6:589:30What Is Radioactive Decay? | Physics in Motion - YouTubeYouTubeStart of suggested clipEnd of suggested clipIt has the same mass as an electron. But has a positive charge. So with beta minus decay a neutronMoreIt has the same mass as an electron. But has a positive charge. So with beta minus decay a neutron decays into a proton and electron.
Why does radioactivity happen?
Radiation is emitted from atoms when an unstable atom decays to become more stable. When an atom has extra neutrons or protons, it causes the element to become unstable.
Why is radioactive decay important?
Scientists and engineers use radioactivity as a source of heat for satellites, for medical imaging, for targeted cancer treatments, for radiometric dating, and for research into the laws of nature and the origin of matter.
Why does radioactivity happen?
Why Are Some Atoms Radioactive? The delicate balance of forces among particles keeps the nucleus stable. Any change in the number, the arrangement, or the energy of the nucleons can upset this balance and cause the nucleus to become unstable and create a radioactive atom.
Why is radioactive decay important?
Scientists and engineers use radioactivity as a source of heat for satellites, for medical imaging, for targeted cancer treatments, for radiometric dating, and for research into the laws of nature and the origin of matter.
What does radioactive decay occur quizlet?
What is radioactive decay? When unstable atoms break down by releasing energy and or particles until they reach a stable form. It is a random process that can be modeled by exponential decay.
Why does a radioactive nuclei decay?
It's basically a matter of thermodynamics. Every atom seeks to be as stable as possible. In the case of radioactive decay, instability occurs when there is an imbalance in the number of protons and neutrons in the atomic nucleus. Basically, there is too much energy inside the nucleus to hold all the nucleons together.
What is beta decay?
Beta Decay is a type of radioactive decay in which a proton is transformed into a neutron or vice versa inside the nucleus of the radioactive sample.
What is a beta decay example?
One of the examples of beta decay is the β – decay of carbon atoms.
What are beta particles?
The beta particle is a high-speed electron when it is a β – decay and a positron when it is a β + decay. For example, the β + decay of carbon-10...
What are the uses of beta decay?
Beta particles are used to treat health conditions such as eye and bone cancer and are also used as tracers.
What happens in beta-plus decay?
In beta plus decay, the proton disintegrates to yield a neutron causing a decrease in the atomic number of the radioactive sample.
What is radioactive decay?
Sample Response: Radioactive decay is the process in which the nucleus of an unstable isotope spontaneously changes, releasing particles and energy. An unstable isotope will continue to decay until it reaches the stable form of either a different isotope of the same element that is stable or a different element that is stable.
What is an alpha decay?
For example, alpha decay is radioactive decay in which an atomic nucleus emits an alpha particle (helium nucleus).
What happens to the nucleus during alpha decay?
Processes like this and alpha decay allow the nucleus of the radioactive sample to get as close as possible to the optimum neutron/ proton ratio. While doing so, the nucleus emits a beta particle which can either be an electron or positron. Remember that there either a proton can turn to a neutron or neutron to a proton.
What is the process by which an unstable atomic nucleus loses energy by radiation?
Radioactivity is the process by which an unstable atomic nucleus loses energy by radiation. The material with unstable nuclei is considered to be radioactive. Nuclear reactions occur randomly. Not all elements undergo nuclear decay over timescales that we can observe. Some elements take millions of years to decay.
What is Beta Decay?
Beta Decay is a type of radioactive decay in which a proton is transformed into a neutron or vice versa inside the nucleus of the radioactive sample. Processes like beta decay and alpha decay allow the nucleus of the radioactive sample to get as close as possible to the optimum neutron/ proton ratio. While doing so, the nucleus emits a beta particle which can either be an electron or positron. Remember that there either a proton can turn to a neutron or neutron to a proton. Electron and the positron are generated to obey the law of conservation of charge. Beta-decay occurs via the weak interaction.
What happens to the neutron in beta minus?
In beta minus, a neutron is transformed to yield a proton causing an increase in the atomic number of the atom. The neutron is neutral but the proton is positive.
How does the nucleus of an atom lose energy?
It is a process by which the nucleus of an unstable atom loses energy by emitting radiation. The nucleus of an atom is held together by the constant balance of two forces; strong nuclear forces of attraction and electrostatic forces of repulsion. These are the two strongest forces in nature.
Can a proton have a beta plus decay?
Beta plus decay can happen only if the daughter nucleus is more stable than the mother nucleus. This difference goes into the conversion of a proton into a neutron, a positron and a neutrino. There is no increase in mass number because a proton and a neutron have the same mass.
Can a proton turn into a neutron?
Remember that there either a proton can turn to a neutron or neutron to a proton. Electron and the positron are generated to obey the law of conservation of charge. The beta decay occurs via the weak interaction. There are two types of beta decay, namely, beta minus (β-) and beta plus (β+).
Answer
Radioactive decay is the process in which the nucleus of an unstable isotope spontaneously changes, releasing particles and energy. An unstable isotope will continue to decay until it reaches the stable form of either a different isotope of the same element that is stable or a different element that is stable.
New questions in Physics
You work in the special effects department of a movie studio. You are currently working on a superhero movie where the hero is very strong and cannot …
How much radiation is absorbed by the atmosphere?
1) 50% of direct and diffused radiation absorbed by land and sea. 2) 5% backscattered to space by atmosphere. 3) 20% reflected from clouds. 4) 20% of radiation absorbed by atmosphere and clouds. 5) 5% reflected from land- sea surface. 6) 30% lost to space by reflection and scattering. Greenhouse effect: three steps.
How does the troposphere absorb solar radiation?
1) absorb solar radiation and re-radiate it as infrared ( long wavelength) radiation. 2) absorbed by Greenhouse gases (water vapor, carbon dioxide, and other trace gases) in the troposphere. 3) causes troposphere to warm= greenhouse effect. - keeps earth livable warmer than if there were no greenhouse effect.
How many atoms are in a container of isotopes?
A container holds 100 atoms of an isotope. This isotope has a half-life of 1.5 months. How many total atoms will be in the container after 3 months?
How many layers of rock are cut by two faults?
Five layers of rock are cut by two faults. Both faults cut through all five layers of rock. Fault A breaks through to the surface, whereas fault B does not. Which of the following statements about faults A and B is most accurate?
Which type of rock is close to horizontal when deposited?
Sedimentary rocks are close to horizontal when deposited.
