
What is the difference between G0 and G1 phases?
While G0 and G1 G-codes are both responsible for the linear movement of the extruder, there is a vital difference that separates one from the other. Despite both these G-codes performing the same action of Linear Move, G0 triggers a movement without extrusion, whereas G1 causes the hotend to extrude plastic as it moves.
Which cells go into the G0 phase?
On occasion, a distinction in terms is made between a G0 cell and a 'quiescent' cell (e.g., heart muscle cells and neurons), which will never enter the G1 phase, whereas other G0 cells may. Cells enter the G0 phase from a cell cycle checkpoint in the G1 phase, such as the restriction point (animal cells) or the start point (yeast).
Do all cells eperience the G0 phase?
G 0 Phase. Not all cells undergo mitotic phase. Cells in the G 0 phase are not actively preparing to divide. The cell is in a quiescent (inactive) stage that occurs when cells exit the cell cycle. Some cells enter G 0 temporarily until an external signal triggers the onset of G 1. No more DNA replication or cell division happens at this phase.
What happens during the G1 and G2 phase?
Initially in G1 phase, the cell grows physically and increases the volume of both protein and organelles. In S phase, the cell copies its DNA to produce two sister chromatids and replicates its nucleosomes. Finally, G2 phase involves further cell growth and organisation of cellular contents. What processes occur during G2 phase?
What are the genes that prevent quiescence?
Functional tumor suppressor genes, particularly p53 and Rb gene , are required to maintain stem cell quiescence and prevent exhaustion of the progenitor cell pool through excessive divisions. For example, deletion of all three components of the Rb family of proteins has been shown to halt quiescence in hematopoietic stem cells. Lack of p53 has been shown to prevent differentiation of these stem cells due to the cells’ inability to exit the cell cycle into the G 0 phase. In addition to p53 and Rb, cyclin dependent kinase inhibitors (CKIs), such as p21, p27, and p57, are also important for maintaining quiescence. In mouse hematopoietic stem cells, knockout of p57 and p27 leads to G 0 exit through nuclear import of cyclin D1 and subsequent phosphorylation of Rb. Finally, the Notch signaling pathway has been shown to play an important role in maintenance of quiescence.
What is the quiescent phase of the cell cycle?
Quiescent stage of the cell cycle in which the cell does not divide. Many mammal cells, such as this 9x H neuron, remain permanently or semipermanently in G 0. The G0 phase describes a cellular state outside of the replicative cell cycle. Classically, cells were thought to enter G 0 primarily due to environmental factors, like nutrient deprivation, ...
What is the transition phase of stem cells?
Stem cell quiescence has been recently suggested to be composed of two distinct functional phases, G 0 and an ‘alert’ phase termed G Alert. Stem cells are believed to actively and reversibly transition between these phases to respond to injury stimuli and seem to gain enhanced tissue regenerative function in G Alert. Thus, transition into G Alert has been proposed as an adaptive response that enables stem cells to rapidly respond to injury or stress by priming them for cell cycle entry. In muscle stem cells, mTORC1 activity has been identified to control the transition from G 0 into G Alert along with signaling through the HGF receptor cMet.
How do stem cells stimulate muscle growth?
Muscle growth can be stimulated by growth or injury and involves the recruitment of muscle stem cells – also known as satellite cells – out of a reversible quiescent state. These stem cells differentiate and fuse to generate new muscle fibers both in parallel and in series to increase force generation capacity.
What is the purpose of myofibers in skeletal muscle?
Skeletal muscle cells continue indefinitely to provide contractile force through simultaneous contractions of cellular structures called sarcomeres. Importantly, these cells are kept in a terminal G 0 phase since disruption of muscle fiber structure after myofiber formation would prevent proper transmission of force through the length of the muscle. Muscle growth can be stimulated by growth or injury and involves the recruitment of muscle stem cells – also known as satellite cells – out of a reversible quiescent state. These stem cells differentiate and fuse to generate new muscle fibers both in parallel and in series to increase force generation capacity.
What is the G0 state?
G 0 was first suggested as a cell state based on early cell cycle studies. When the first studies defined the four phases of the cell cycle using radioactive labeling techniques, it was discovered that not all cells in a population proliferate at similar rates. A population's “growth fraction” – or the fraction of the population that was growing – was actively proliferating, but other cells existed in a non-proliferative state. Some of these non-proliferating cells could respond to extrinsic stimuli and proliferate by re-entering the cell cycle. Early contrasting views either considered non-proliferating cells to simply be in an extended G 1 phase or in a cell cycle phase distinct from G 1 – termed G 0. Subsequent research pointed to a restriction point (R-point) in G 1 where cells can enter G 0 before the R-point but are committed to mitosis after the R-point. These early studies provided evidence for the existence of a G 0 state to which access is restricted. These cells that do not divide further exit G1 phase to enter an inactive stage called quiescent stage.
How does miRNA regulate gene expression?
Post-transcriptional regulation of gene expression via miRNA synthesis has been shown to play an equally important role in the maintenance of stem cell quiescence. miRNA strands bind to the 3’ untranslated region ( 3’ UTR) of target mRNA ’s, preventing their translation into functional proteins. The length of the 3’ UTR of a gene determines its ability to bind to miRNA strands, thereby allowing regulation of quiescence. Some examples of miRNA's in stem cells include miR-126, which controls the PI3K/AKT/mTOR pathway in hematopoietic stem cells, miR-489, which suppresses the DEK oncogene in muscle stem cells, and miR-31, which regulates Myf5 in muscle stem cells. miRNA sequestration of mRNA within ribonucleoprotein complexes allows quiescent cells to store the mRNA necessary for quick entry into the G1 phase.
What are the effects of MSCs on T cells?
Di Nicola M et al. reported that human bone marrow MSCs inhibit both CD4 + and CD8 + T-lymphocyte proliferation by secreting transforming growth factor beta 1 (TGFβ1), hepatocyte growth factor (HGF), and prostaglandin E 2 (PGE 2) in vitro [ 7 ]. Another study showed that MSCs inhibit stimulated lymphocyte proliferation and mitogenic response independently of the major histocompatibility complex (MHC) [ 8 ]. MSCs also produce indoleamine 2,3-dioxygenase (IDO), which accelerates tryptophan degradation and kynureine synthesis resulting in inhibition of T-lymphocyte proliferation [ 9 ]. Nitric oxide (NO) is another immune regulation factor secreted by MSCs [ 10, 11 ]. NO inhibits proliferation of T-lymphocytes by suppressing phosphorylation of transcription factor, signal transducer, and activator transcription-5 (STAT-5) [ 12 ]. Human leucocyte antigen-G5 (HLA-G5) from MSCs is a trigger for inhibition of T-lymphocyte function, followed by up-regulation of T-helper type 2 (Th2) and regulatory T-cell (Tregs) [ 13, 14 ]. On the other hand, MSCs are able to inhibit T-lymphocyte proliferation by direct cell-to-cell contact [ 15–17 ]. Krampera et al. reported that MSCs physically hinder T-lymphocytes from contacting antigen presenting cells in a non-cognate fashion [ 18 ].
What is the role of miR 27b in breast cancer?
Recent findings by Li et al. [57] suggest that miR-27b (microRNA 27b) promoter methylation regulates tamoxifen sensitivity in breast cancer cells. MiR-27b downregulation has been associated with tamoxifen resistance in breast cancer cells [58], and the study by Li et al. [57] showed that this downregulation results from promoter methylation. They furthermore showed that cells with enforced miR-27b expression were sensitized to tamoxifen treatment. The authors demonstrated that the miR-27b effect was mediated through regulation of the HMGB3 gene, which has known involvement in drug resistance. HMGB3 expression was found to be upregulated in tamoxifen-resistant breast cancer cells and decreased when miR-27b was transfected into these cells. Additional findings suggest that other microRNAs regulate HMGB3 not only in breast cancer [59] but also in gastric cancer [60].
What is the effect of tamoxifen on cancer?
Tamoxifen is a potent nonsteroidal estrogen receptor antagonist hindering cancer cell growth at the G 0 and G 1 phase . Tamoxifen is used for 10 conditions including its label indications for metastatic breast cancer and adjuvant treatment of breast cancer after primary treatment with surgery and radiation. The ESR1 gene encodes estrogen receptor α, a nuclear hormone receptor. Upon activation by estrogen, ESR1 functions as a transcription factor vital for multiple processes including sexual and reproductive development, bone density, and development of breast and endometrial cancer. High ESR1 methylation at the transcription start site of exon 1A was associated with tamoxifen response in a 2004 study involving 148 breast cancer patients [56]. ESR1 was found to be a significant predictor of survival and tamoxifen response in the tamoxifen-treated group and had no predictive power in the nontamoxifen group.
How do MSCs induce apoptosis?
A previous study demonstrated that MSCs secrete IDO, induce 3-Hydroxyanthranilic acid (HAA) synthesis during tryptophan metabolism, and induce cell apoptosis by inhibiting the NF κB pathway in T-lymphocytes [ 21 ]. Augello et al. reported that MSCs induce apoptosis of T-lymphocytes by activation of the programmed death 1 pathway [ 22 ]. More recently, MSCs have been demonstrated to induce T-lymphocyte apoptosis through the FAS/FAS ligand (FASL) pathway, and consequently lead to immunotolerance ( Figure 61.1) [ 23 ].
How does a lymphocyte convert into a lymphoblast?
Binding of specific antigen activates a lymphocyte and stimulates it to progress through cell division. The transcription of numerous genes is triggered, causing the progeny cells to undergo morphologic and functional changes that result in the production of lymphoblasts. This conversion of the resting lymphocyte into a lymphoblast occurs within 18–24 hours after antigen receptor activation. Lymphoblasts are larger and display more cytoplasmic complexity than their resting cell counterparts. They undergo rapid cell division and differentiation into long-lived memory and short-lived effector cells; in fact, effector cells can be detected after the first cell division. For B cells, the fully differentiated effector cell is an antibody-secreting plasma cell. T cell effectors of the helper type (Th) become cytokine secretors, while those of the cytotoxic type (Tc) develop a mechanism allowing them to directly lyse target cells.
What is the class of resting B and T lymphocytes?
Resting B and T lymphocytes (those in the G 0 phase of the cell cycle) are classed histologically as “small lymphocytes” (refer to Fig. 3-1 ). Morphologically, small lymphocytes are round cells with a large nucleus surrounded by a narrow rim of cytoplasm. Comparatively few intracellular organelles are contained in the cytoplasmic rim. Resting B and T cells that have not interacted with specific antigen are said to be virgin, naive, or unprimed. They have a short life span (up to a few weeks) and undergo apoptosis unless they encounter their specific antigen.
What is a small lymphocyte?
Morphologically, small lymphocytes are round cells with a large nucleus surrounded by a narrow rim of cytoplasm. Comparatively few intracellular organelles are contained in the cytoplasmic rim. Resting B and T cells that have not interacted with specific antigen are said to be virgin, naive, or unprimed.
What happens in the G0 phase?
Not all cells undergo mitotic phase. Cells in the G0 phase are not actively preparing to divide. The cell is in a quiescent (inactive) stage that occurs when cells exit the cell cycle. Some cells enter G0 temporarily until an external signal triggers the onset of G1. No more DNA replication or cell division happens at this phase. The cells that never or rarely divide include mature cardiac muscle and nerve cells, and they remain in G0 permanently.
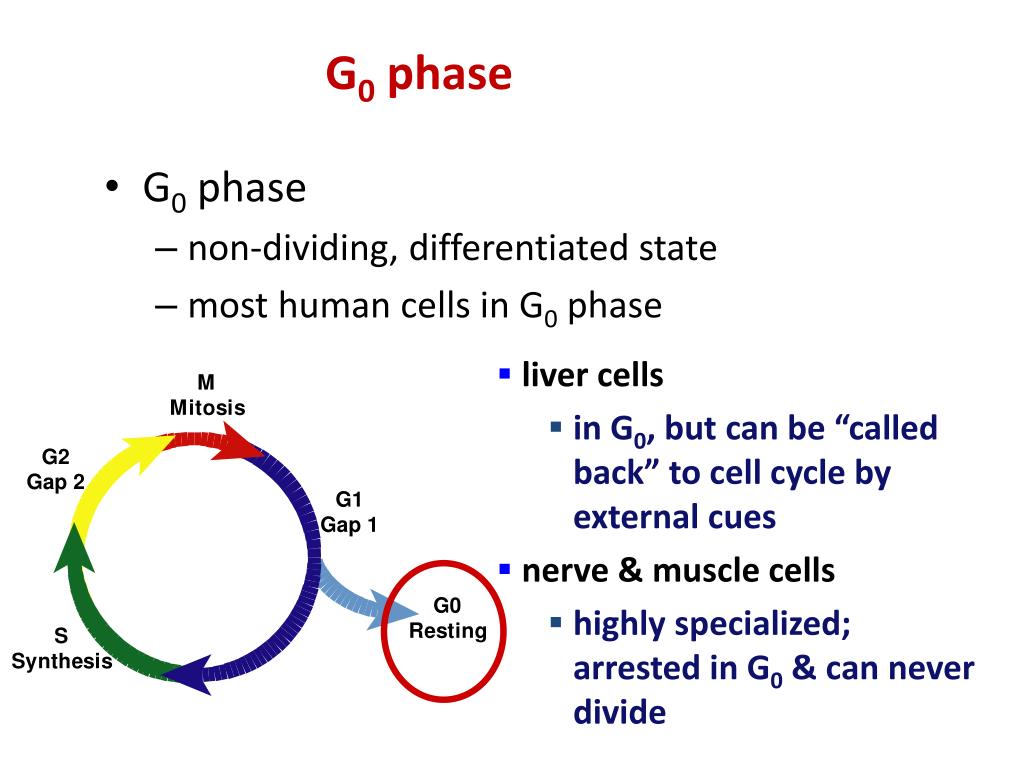
What are the stages of the cell cycle?
The Stages of Interphase and the Cell Cycle: The cell cycle consists of interphase and the mitotic phase. During interphase, the cell grows and the nuclear DNA is duplicated. Interphase is followed by the mitotic phase. During the mitotic phase, the duplicated chromosomes are segregated and distributed into daughter nuclei. The cytoplasm is usually divided as well, resulting in two daughter cells
What is the stage of the life cycle where the cell grows and DNA is replicated?
interphase:the stage in the life cycle of a cell where the cell grows and DNA is replicated. centrosome:an organelle near the nucleus of a cell that contains the centrioles (in animal cells) and from which the spindle fibers develop in cell division.
What are the two centrosomes?
The two centrosomesgive rise to themitotic spindle, the apparatus that orchestrates the movement of chromosomes during mitosis. At the center of each animal cell, the centrosomes of animal cells associate with a pair of rod-like objects, the centrioles, which are at right angles to each other.
What is the function of a cell in replicating chromosomes?
The cell grows and accumulates the building blocks of chromosomal DNA and the associated proteins as well as sufficient energy reserves to complete the task of replicating each chromosome in the nucleus. Cells increase in size and produce organelles. The cell has two choices at this point: to divide or not to divide.
What is the first stage of interphase?
The first stage of interphase is the G1 phase (first gap), the growing phase. All cells undergo G1. Here, the cell is quite active at the biochemical level. The cell grows and accumulates the building blocks of chromosomal DNA and the associated proteins as well as sufficient energy reserves to complete the task of replicating each chromosome in the nucleus. Cells increase in size and produce organelles.
Which phase of interphase takes the longest?
These cells do not go through S or G2. They stop at G1 or G0. S Phase (Synthesis of DNA) The synthesis phase of interphase takes the longest because of the complexity of the duplicated genetic material. The S phase is where DNA replication occurs, and centrioles replicate.
Overview
The G0 phase describes a cellular state outside of the replicative cell cycle. Classically, cells were thought to enter G0 primarily due to environmental factors, like nutrient deprivation, that limited the resources necessary for proliferation. Thus it was thought of as a resting phase. G0 is now known to take different forms and occur for multiple reasons. For example, most adult n…
Diversity of G0 states
Three G0 states exist and can be categorized as either reversible (quiescent) or irreversible (senescent and differentiated). Each of these three states can be entered from the G1 phase before the cell commits to the next round of the cell cycle. Quiescence refers to a reversible G0 state where subpopulations of cells reside in a 'quiescent' state before entering the cell cycle after activation in response to extrinsic signals. Quiescent cells are often identified by low RNA conten…
Characteristics of quiescent stem cells
The transcriptomes of several types of quiescent stem cells, such as hematopoietic, muscle, and hair follicle, have been characterized through high-throughput techniques, such as microarray and RNA sequencing. Although variations exist in their individual transcriptomes, most quiescent tissue stem cells share a common pattern of gene expression that involves downregulation of cell cycle progression genes, such as cyclin A2, cyclin B1, cyclin E2, and survivin, and upregulation of …
Regulation of quiescence
Functional tumor suppressor genes, particularly p53 and Rb gene, are required to maintain stem cell quiescence and prevent exhaustion of the progenitor cell pool through excessive divisions. For example, deletion of all three components of the Rb family of proteins has been shown to halt quiescence in hematopoietic stem cells. Lack of p53 has been shown to prevent differentiation of these stem cells due to the cells’ inability to exit the cell cycle into the G0 phase. In addition to p…
Examples of reversible G0 phase
Stem cells are cells with the unique ability to produce differentiated daughter cells and to preserve their stem cell identity through self-renewal. In mammals, most adult tissues contain tissue-specific stem cells that reside in the tissue and proliferate to maintain homeostasis for the lifespan of the organism. These cells can undergo immense proliferation in response to tissue damage before differentiating and engaging in regeneration. Some tissue stem cells exist in a re…
Examples of irreversible G0 phase
Often associated with aging and age-related diseases in vivo, senescent cells can be found in many renewable tissues, including the stroma, vasculature, hematopoietic system, and many epithelial organs. Resulting from accumulation over many cell divisions, senescence is often seen in age-associated degenerative phenotypes. Senescent fibroblasts in models of breast epithelial cell function have been found to disrupt milk protein production due to secretion of matrix metall…
Mechanism of G0 entry
Rim15 was first discovered to play a critical role in initiating meiosis in diploid yeast cells. Under conditions of low glucose and nitrogen, which are key nutrients for the survival of yeast, diploid yeast cells initiate meiosis through the activation of early meiotic-specific genes (EMGs). The expression of EMGs is regulated by Ume6. Ume6 recruits the histone deacetylases, Rpd3 and Sin3, to repress EMG expression when glucose and nitrogen levels are high, and it recruits the EMG tr…
Mechanism of G0 exit
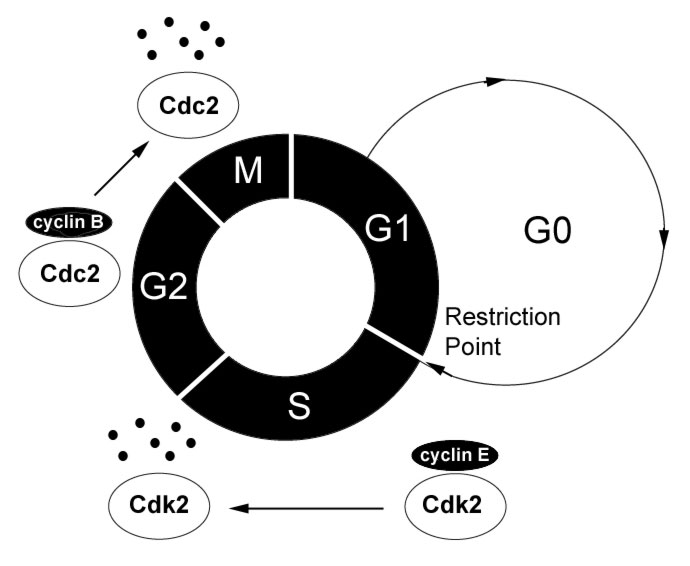
The transition from G1 to S phase is promoted by the inactivation of Rb through its progressive hyperphosphorylation by the Cyclin D/Cdk4 and Cyclin E/Cdk2 complexes in late G1. An early observation that loss of Rb promoted cell cycle re-entry in G0 cells suggested that Rb is also essential in regulating the G0 to G1 transition in quiescent cells. Further observations revealed that levels of cyclin C mRNA are highest when human cells exit G0, suggesting that cyclin C may …