What happens when you mix acid and base?
What is the difference between acid and base?
Why is water not formed?
What is the reaction between HCl and NaOH?
What gases are produced when you mix vinegar and baking soda?
Is mixing an acid with a base a chemical reaction?
Is salt soluble in water?
See 4 more
About this website
What happens when a strong acid reacts with a strong base?
In addition, the anion (negative ion) created from the dissociation of the acid combines with the cation (positive ion) created from the dissociation of the base to create a salt. Therefore, the reaction between a strong acid and strong base will result in water and a salt.
What happens when you mix a base and an acid together?
When an acid and a base are placed together, they react to neutralize the acid and base properties, producing a salt.
What happens when a strong acid and weak base are mixed?
In a weak base-strong acid titration, the acid and base will react to form an acidic solution. A conjugate acid will be produced during the titration, which then reacts with water to form hydronium ions. This results in a solution with a pH lower than 7.
Why should you never combine a strong acid and a strong base?
If acids and bases mix, it can result in violent neutralisation reactions. For example; if a strong acid such as hydrochloric acid mixed with a strong base such as sodium hypochlorite, it would result in a violent chemical reaction that would produce a lot of heat and gas.
What is it called when you mix an acid and a base?
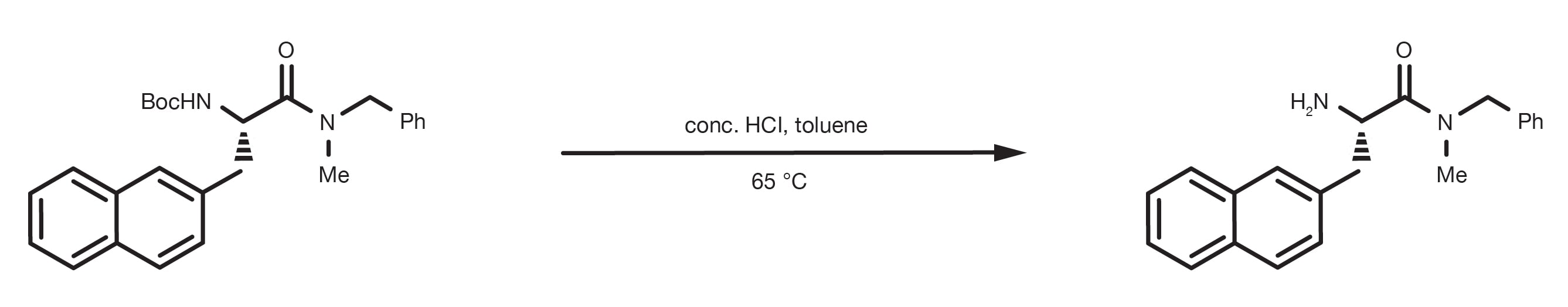
The reaction of an acid with a base is called a neutralization reaction. The products of this reaction are a salt and water.
When an acid reacts with a strong base which product always forms?
So now that we know what they are, what do we do with them? Well, whenever an acid and a hydroxide base are reacted together they always produce the same two types of products: a salt and water.
Is it safe to add acid to base?
Mixing acids and bases (even just in water) is ALWAYS DANGEROUS, in any quantity, and SAFETY should be your first concern.
Can acids and bases Cannot mix together?
Acids and bases cannot mix together. Acids and bases will neutralize each other. Acids, but not bases, can change the pH of a solution. Acids donate hydroxide ions (OH–); bases donate hydrogen ions (H+).
When a solution of an acid and a base are mixed together quizlet?
A neutralization reaction is when an acid and a base react to form water and a salt and involves the combination of H+ ions and OH- ions to generate water. The neutralization of a strong acid and strong base has a pH equal to 7.
What happens to an acid and base in a water solution?
Hence, acids and bases mix in water to dissociate and form hydronium and hydroxide ions respectively. Additional information: Acids and bases react together to form salts. This is called a neutralization reaction, and can yield various important salts, like sodium chloride, as HCl+NaOH→NaCl+H2O .
What are the two products of a mixture of an acid and base?
Most introductory chemistry books will teach that the reaction between an acid and a base is called neutralization, and the products formed are water and a salt.
Why can the mixing of acids and bases be dangerous
Yes, the practical message is you need to be cautious, protected, supervised etc. when playing with such things or not do it at all. It is all, as Studiot et al. say to do with energy. Just complete that by saying that these strong acids and bases (and a large fraction of the things you will study in chemistry) are only there for anyone to play with because energy has been put in to make them ...
Do you add an acid to a base or a base to an acid? - Answers
What happens if you add an acid to a base? If you add the same quantity of an acid and a base it will become neutral.i.e forms salt and water
How do you safely mix acids and bases? | Socratic
See below. Acids and bases can both be extremely dangerous. Remember that acids and bases can both be corrosive and/or irritating to human skin, so make sure not to make skin contact. Wear safety goggles at all times when dealing with acids and bases, and your teacher may even instruct you to put on gloves. If you make eye or skin contact with an acid or base, notify your teacher immediately ...
What Happens When you Mix an Acid with a Base? Kids Science
Experiment 2 Vinegar and Orange Juice. In this experiment you will find out what happens when you mix and acid with a base. You will be using vinegar and orange juices as acids and baking soda as a base.
What Happens When We Mix Acid with Base Solutions?
Disclaimer and Safety Precautions Education.com provides the Science Fair Project Ideas for informational purposes only. Education.com does not make any guarantee or representation regarding the Science Fair Project Ideas and is not responsible or liable for any loss or damage, directly or indirectly, caused by your use of such information.
Answer
When they are equally strong they will both neutralize each other, and the acidic and basic properties are no longer there.
New questions in Chemistry
Jason wants to monitor the temperature of water as it heats on the stove. Which tool should he use to measure the water's temperature? A a balance B …
What happens when you mix acid and base?
If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other out and produce a salt and water. Mixing equal amounts of a strong acid with a strong base also produces a neutral pH (pH = 7) solution. This is called a neutralization reaction and looks like this:
What is the difference between acid and base?
First, it helps to understand what acids and bases are. Acids are chemicals with a pH less than 7 that can donate a proton or H + ion in a reaction. Bases have a pH greater than 7 and can accept a proton or produce an OH - ion in a reaction. If you mix equal amounts of a strong acid and a strong base, the two chemicals essentially cancel each other ...
Why is water not formed?
Also, water may not be formed because most weak bases are not hydroxides (no OH - available to form water).
What is the reaction between HCl and NaOH?
An example would be the reaction between the strong acid HCl (hydrochloric acid) with the strong base NaOH (sodium hydroxide): HCl + NaOH → NaCl + H 2 O + heat. The salt that is produced is table salt or sodium chloride. Now, if you had more acid than base in this reaction, not all of the acid would react, so the result would be salt, water, ...
What gases are produced when you mix vinegar and baking soda?
Sometimes gases are produced. For example, when you mix baking soda (a weak base) with vinegar (a weak acid), you get carbon dioxide. Other gases are flammable, depending on the reactants, and sometimes these gases are flammable, so you should use care when mixing acids and bases, especially if their identity is unknown.
Is mixing an acid with a base a chemical reaction?
Anne Marie Helmenstine, Ph.D. Updated September 19, 2018. Mixing an acid with a base is a common chemical reaction. Here is a look at what happens and the products resulting from the mixture.
Is salt soluble in water?
Other salts are not soluble in water, so they form a solid precipitant. In either case, it's easy to see the acid and base were neutralized. Test your understanding with an acids and bases quiz . Helmenstine, Anne Marie, Ph.D. "Acid-Base Chemical Reaction.".