Is methanol more acidic than ethanol?
Yes,methanol is more acidic than ethanol or we can also say ethanol is more basic than methanol due to its molar mass. Both ethanol and methanol have OH functional group.Then how we are going to determine its acidity or basicity?
Will NaOH react with ethanol?
Sodium ethoxide can be made by reacting sodium metal or sodium hydride with absolute ethanol. As you mentioned, NaOH does not react with 95% or 100% ethyl alcohol since NaOH is not efficient (strong enough) base to abstract a proton from ethanol (this proton is not acidic at all, it is almost neutral).
Does ethanol react with acid?
Ethanol to ethanoic acid reaction. Ethanol reacts with strong oxidizing agents such as H + / KMnO 4, H + / K 2 CrO 4, H + / K 2 Cr 2 O 7 to give ethanoic acid. Ethanoic acid to ethanol reaction. Ethanoic acid reacts with LiAlH 4 / ether to give ethanol. Ethanol and ethanoic acid chemical properties. Ethanol is an alcohol compound.
What is the reaction between ethanol and dichloromethane?
They simply mix together. There is NO reaction between the two solvents. By the way the abbreviation of dichloromethane is DCM and not MDC. Hoping this will be helpful, Rafik. Cite. 18th Mar, 2016...

What type of reaction occurs when ethanol reacts with ethanoic acid?
Alcohols and carboxylic acids react and give esters. This reaction is catalyzed by concentrated sulfuric acid. Ethanol reacts with acetic acid to give ethyl ethanoate which is an ester compound.
What happens when ethanol reacts with acid?
By the reaction of ethanol with acetic acid in the presence of sulphuric acid, form an ester which is known as ethyl acetate. The sulphuric acid will help with the elimination of water. And when ethanol is heated with concentrated sulphuric acid, the ethanol gets dehydrated and forms an unsaturated hydrocarbon.
When ethanol reacts with ethanoic acid in the presence of concentrated H2SO4 equation?
Solution : When ethanol reacts with ethanoic acid on warming with conc. `H_(2)SO_(4)`, sweet smelling ester is formed.
`CH_(3)COOH + C_(2)H_(5)OH rarr underset("Ester")(CH_(3)COOC_(2)H_(5))+H_(2)O`
The name of the product is ethyl ethanoate.
What are the properties of ethanol and ethanoic acid?
EthanolEthanoic acidIt has a burning taste.It has a sour taste.Ethanol exist as a liquid at room temperature, and it has a melting point of 156K and a boiling point of 351 K.Ethanoic acid has a melting point as 16oC. It often freezes in the winter season when the climate is cold.(Chemical properties)3 more rows
What is esterification reaction explain using ethanol and ethanoic acid as examples?
For example, ethanol reacts with ethanoic acid in the presence of an acid catalyst to give sweet smelling substance, i.e. an ester which in this case will be ethylethanoate. CH3-COOH + CH3-CH2-OH →Acid CH3-COO-CH2-CH3 + H2O. Ethanoic acid Ethanol Ethyl ethanoate (Ester) This reaction is known as esterification reaction ...
What is the smell of ethanol mixed with ethanoic acid?
fruity smellWhen ethanol reacts with ethanoic acid in the presence of Conc. H2SO4, a substance with fruity smell is produced.
What is the smell of ethanoic acid and ethanol?
Ethanol is a colourless, volatile, flammable liquid with a wine-like odour and a strong flavour. Ethanoic acid, often known as acetic acid, is a harsh, acrid-smelling chemical. You could know the fragrance as vinegar-like.
Why is the conversion of ethanol to ethanoic acid an oxidation reaction?
The conversion of ethanol to ethanoic acid involves the removal of the Hydrogen atom and the addition of oxygen (oxidation reaction). In the first step, the Hydrogen molecule is removed from ethanol to form ethanal. Since Hydrogen loss is oxidation so, the reaction is an oxidation reaction.
What happens when alcohol is treated with HCl?
When treated with HBr or HCl alcohols typically undergo a nucleophilic substitution reaction to generate an alkyl halide and water. Alcohol relative reactivity order : 3o > 2o > 1o > methyl. Hydrogen halide reactivity order : HI > HBr > HCl > HF (paralleling acidity order).
What happens when alcohol reacts with carboxylic acid?
In the presence of acid, a carboxylic acid and an alcohol react to form ester.
What happens when ethanol reacts with ethanol acid in presence of sulphuric acid?
A reminder of the facts Ethanoic acid reacts with ethanol in the presence of concentrated sulphuric acid as a catalyst to produce the ester, ethyl ethanoate. The reaction is slow and reversible.
What happen when ethanol react with water?
There is no addition reaction with butanol because it has no π -bonds anywhere.
What is the difference between ethanol and ethanoic acid?
There are various differences between ethanol and ethanoic acid. Ethanol has an ethyl group, while ethanoic acid has a methyl group as a substituen...
What happens when ethanoic acid reacts with Ethanol?
Ethanol reacts with ethanoic acid to produce a compound with a fruity smell is called Ethyl ethanoate which is an ester. This reaction is known as...
What does Ethanol react with to make ethanoic acid?
Ethanol on oxidation from Ethanoic acid in the presence of a strong oxidising agent like acidified KMnO4
What is the formula of Ethanol?
The chemical formula of Ethanol is C2H5OH. Ethanol is prepared by fermentation of sugars by yeast.
What is the smell of ethanol and ethanoic acid?
Ethanol is a volatile, colourless, flammable liquid with a pleasant smell, while ethanoic acid is a colourless liquid with a strong, vinegar-like s...
What are the uses of Ethanol and Ethanoic Acid?
Ethanol is used in the pharmaceutical industry to manufacture medicines like tincture of iodine (antiseptic that contains2% iodine, along with pota...
What is the effect of oxidation on ethanol?
Ethanol on oxidation leads to the formation of Ethanoic acid in the presence of a strong oxidising agent like potass ium permanganate under acidic conditions.
What is the reaction between ethanoic acid and sodium carbonate?
Ethanoic acid reacts with sodium carbonate and sodium bicarbonate to form a salt, water, and carbon dioxide gas. This reaction can be confirmed by a laboratory experiment in which effervescence is observed in a test tube having sodium carbonate or sodium bicarbonate when acetic acid is added. When the gas released in effervescence is passed through the lime water, it turns milky due to the formation of calcium carbonate precipitate. This confirms the release of carbon dioxide gas. This property of carbonates and bicarbonates to react with acid and release carbon dioxide gas is used in baking cakes.
What happens when sodium is dropped into a container of ethanoic acid?
When a small piece of Sodium is dropped into a container of ethanoic acid, it reacts steadily to form a colourless solution of sodium acetate and gives off bubbles of hydrogen gas. It is an excellent example of an acid-base reaction where sodium acts as a base and ethanoic acid.
What is the chemical formula for ethanol?
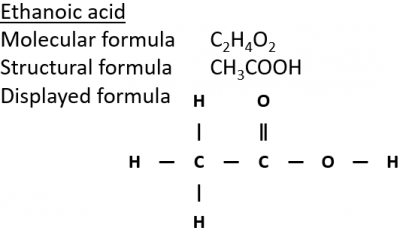
The chemical formula of Ethanol is C 2 H 5 O H , and that of ethanoic acid is C H 3 C O O H. Ethanol is prepared by fermentation of sugars by yeast. It is used in making alcoholic beverages, neurotoxin drugs, etc. Ethanol is a volatile, colourless, flammable liquid with a pleasant smell. Ethanoic acid is a colourless liquid having a strong, pungent smell commonly known as acetic acid. Ethanol is oxidised to ethanoic acid in the presence of a strong oxidising agent.
What is the reverse of the esterification reaction in which acidic hydrolysis takes place?
Saponification: It is the reverse of the esterification reaction in which acidic hydrolysis takes place. Water and ester salt is used to produce back carboxylic acid and alcohol.
What is the reaction of alcohol to alkene?
Dehydration of alcohol is a famous reaction to form an alkene. Ethanol, when heated to around 170 ∘ C in the presence of an excess of sulphuric acid, it gives ethene and water is released. In this reaction, sulphuric acid acts as a catalyst.
Is ethanoic acid a liquid?
On the other hand, ethanoic acid is also a colourless liquid. It is commonly known as ‘acetic acid’. Its aqueous solution turns blue litmus red, showing that it is acidic. The functional group present in ethanoic acid is ‘carboxylic acid’. In this article, let’s learn about ethanol and ethanoic acid in detail.
