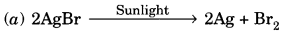
What happens when carbon monoxide reacts with iron(III) oxide?
Not all the iron (III) oxide is reduced by carbon monoxide in this way.We call this oxidation, because each molecule of carbon monoxide gains an oxygen atom. The overall process is a redox reaction, in which iron (III) oxide is reduced and carbon monoxide is oxidised. Not all the iron (III) oxide is reduced by carbon monoxide in this way.
What happens when you mix carbon dioxide and carbon monoxide?
Carbon dioxide can react with carbon to form carbon monoxide by the following equilibrium equation: The reaction to the right is endothermic, which means that at higher temperature more CO will be formed. If we can just produce iron from iron oxide by reacting it with carbon monoxide, why do we use blast furnaces?
Which reaction is used in iron smelting?
Background Info: This reaction is used in Iron smelting basically iron extraction because here oxygen is an impurity and carbon is added to form iron and carbon dioxide. This in turn forms carbon monoxide but still it purifies iron. Iron is Fe2O3 right??
How does Feo react with carbon monoxide in a blast furnace?
Fe2+ in FeO has a lower tendency to be reduced but it reacts with carbon monoxide in a blast furnace to produce steel. The high temperature causes the carbon to burn in oxygen and produce CO (carbon monoxide). The CO then reacts with FeO as follows:
What happens when ferric oxide is heated with carbon monoxide?
Iron (III) oxide on heating with carbon monoxide gas form solid iron and liberates carbon dioxide gas.
What is the chemical equation for the reaction between iron oxide and carbon monoxide?
Fe2O3 + CO → Fe + CO2 - Chemistry Q&A.
How do you balance fe2 o3 co Fe CO2?
0:003:33Balance Fe2O3 + CO = Fe + CO2 --- Iron (III) Oxide + Carbon MonoxideYouTubeStart of suggested clipEnd of suggested clipWe have two iron atoms we have three oxygen atoms here plus the one. Here. So we have four oxygenMoreWe have two iron atoms we have three oxygen atoms here plus the one. Here. So we have four oxygen atoms. And then we have the one carbon on the product side we have one iron two oxygens.
What type of reaction is Fe2O3 Co?
0:011:58Type of Reaction for Fe2O3 + CO = Fe + CO2 - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo when we look down our table here's our single replacement reaction. And for the singleMoreSo when we look down our table here's our single replacement reaction. And for the single replacement reaction follows this format a plus bc yields b plus ac.
Why is iron oxide and carbon monoxide a redox reaction?
Most of the iron (III) oxide is reduced using carbon monoxide gas. This gas is a reducing agent which takes the oxygen away from iron (III) oxide. Notice that carbon monoxide gas in this reaction is changing into carbon dioxide. We call this oxidation, because each molecule of carbon monoxide gains an oxygen atom.
Is Fe2O3 a reducing agent?
∵Fe2O3 is undergoing reduction, so it acts as oxidising agent.
What is the balanced equation for Fe o2 Fe2O3?
0:001:45Balancing Fe + O2 = Fe2O3 (Iron + Oxygen gas) - YouTubeYouTubeStart of suggested clipEnd of suggested clipTo balance this chemical equation we have Fe plus o2 yields fe2o3. This is iron plus oxygen. AndMoreTo balance this chemical equation we have Fe plus o2 yields fe2o3. This is iron plus oxygen. And this is iron to oxide.
Is Fe2O3 3CO 2Fe 3CO2 a balanced equation?
“There is no detectable change in the quantity of matter during an ordinary chemical reaction.” The above equation balances as: Fe2O3 + 3CO → 2Fe + 3CO2 The balanced equation must obey the Law of Conservation of Matter.
What is the formula for iron LLL oxide?
Fe₂O₃Iron(III) oxide / Formula
Is it possible to produce iron by Fe2O3 with CO?
Iron (III) oxide can be reduced with CO to form metallic iron as described by unbalanced chemical reaction. Fe2O3+CO→Fe+CO2.
What type of reaction is FeO co?
0:001:06How to Balance FeO + CO = Fe + CO2 - YouTubeYouTubeStart of suggested clipEnd of suggested clipIn this video we'll bounce the equation FeO plus Co that's iron to oxide plus carbon monoxide.MoreIn this video we'll bounce the equation FeO plus Co that's iron to oxide plus carbon monoxide.
What is the balanced equation for Fe o2 fe2o3?
0:001:45Balancing Fe + O2 = Fe2O3 (Iron + Oxygen gas) - YouTubeYouTubeStart of suggested clipEnd of suggested clipTo balance this chemical equation we have Fe plus o2 yields fe2o3. This is iron plus oxygen. AndMoreTo balance this chemical equation we have Fe plus o2 yields fe2o3. This is iron plus oxygen. And this is iron to oxide.
How do you balance pb3o4 PBO o2?
0:461:47How to Balance Pb3O4 = PbO + O2 - YouTubeYouTubeStart of suggested clipEnd of suggested clipNumbers. So we have 6 times 1 6 lead atoms and then 6 times 1 6 plus the 2 that'll give us 8 oxygenMoreNumbers. So we have 6 times 1 6 lead atoms and then 6 times 1 6 plus the 2 that'll give us 8 oxygen atoms all we need to do here now is just double. This 3 times 2 that equals 6.
What is the formula for iron LLL oxide?
Fe₂O₃Iron(III) oxide / Formula
What is the chemical formula of carbon monoxide?
COCarbon monoxide / Formula
Answer
Option D. For every mole of iron oxide consumed, two moles of iron are produced.
New questions in Chemistry
Which statement is a hypothesis? A- crickets are cooler than grasshoppers B- if the temperature increases, then crickets will chirp more. C- I hear c …
How many mol of Fe2O3 reacts with 3/1)?
Therefore, 0.6011 mol of Fe2O3 reacts with (3/1) x 0.6011 = 1.8033 mol of CO to produce iron and CO.
What happens if air is insufficient to produce CO2?
Wait a minute. Carbon burns in oxygen to produce CO2; if air is insufficient it will produce CO or carbon monoxide. C + O2 → CO2. Now if there is insufficient oxygen or more usually the fire can’t get to it we get 2C + O2 → 2 CO.
How many moles of Fe2O3 are needed to produce 500 g of Fe?
Hence, the number of mole of Fe2O3 required to produce 500 g of Fe = 4.477 mol.
How many moles of Fe are produced from 1 mole of Fe2O3?
From the balanced equation, 2 moles of Fe are produced from 1 mole of Fe2O3 reacting with CO.
How to find the valency of iron?
For solving this first of all you figure out the formula of iron (3)oxide so you look at the periodic table as you said valency of iron is 3 positive because metals are always positive and oxygen’s valency is 2 (negative because non-metal). You criss-cross both you take 2 from oxygen and put it under iron and then 3 from iron under oxygen.
What is the valency of iron?
For solving this first of all you figure out the formula of iron (3)oxide so you look at the periodic table as you said valency of iron is 3 positive because metals are always positive and oxygen’s valency is 2 (negative because non-metal). You criss-cross both yo
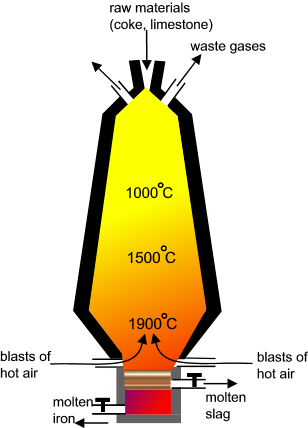
What is the reaction that takes place in a blast furnace?
This is the classic Boudouard reaction that takes place in a blast furnace. Iron ore Fe2O3 is reduced by CO gas to produce Fe and CO2.
.png)