
When vapor pressure of liquid becomes equal to the external pressure?
“When vapor pressure of liquid becomes equal to the external pressure, the liquid starts boiling” Vapor pressure, in the simplest words, can be defined as “the equilibrium pressure exerted by the vapors on the surface of liquid at a given temperature.”
What is the relationship between vapor pressure and boiling point?
Given that the boiling point of a liquid is the temperature at which the vapor pressure is equal to the ambient (surrounding) pressure, what significance does a liquid's vapor pressure have in the formation of bubbles that happens at and above the boiling point?
Why does external hydrostatic pressure increase vapor pressure?
That's why a higher temperature is required to increase the vapor pressure. However, the online source makes the case that external hydrostatic pressure increases solvent molecules' tendency to escape from the liquid phase into the vapor phase. I'm confused as to why more gases would escape when there is an external pressure on the liquid....
Why does vapor pressure increase with temperature?
This pressure at that particular temperature is known as vapor pressure. As the temperature increases, the kinetic energy of molecules also increase and more and more molecules escape from the bounds of intermolecular forces. Which means, more molecules enter into vapor phase and thus more is the vapor pressure.
What will happen if the atmospheric or external pressure is equal to the vapor pressure of a liquid?
If the liquid is in an open container and exposed to normal atmospheric pressure, the liquid boils when its saturated vapor pressure becomes equal to 1 atmosphere (or 101325 Pa or 101.325 kPa or 760 mmHg). This happens with water when the temperature reaches 100°C.
What happens when the vapor pressure of water equals?
The water vapor pressure is the partial pressure of water vapor in any gas mixture in equilibrium with solid or liquid water. Like all liquids, water boils when the vapor pressure is equal to the surrounding pressure.
When the vapor pressure is equal to the atmospheric pressure?
boiling pointBoiling will occur when the vapor pressure is equal to the atmospheric pressure. This is called the boiling point. Without any external pressure the liquid molecules will be able to spread out and change from a liquid to a gas.
Why does boiling occur when vapor pressure equals atmospheric pressure?
When any substance in the liquid phase, at atmospheric pressure, it means that this pressure is sufficient to confine the molecules together to form liquid. The molecules should possess equal energy to overcome this pressure for the liquid to boil.
At what temperature is the pressure of the vapor in a liquid equal to the external pressure on that liquid?
boiling temperatureThe temperature at which vapour pressure of a liquid is equal to the external pressure is called boiling temperature.
Does higher vapor pressure mean faster evaporation?
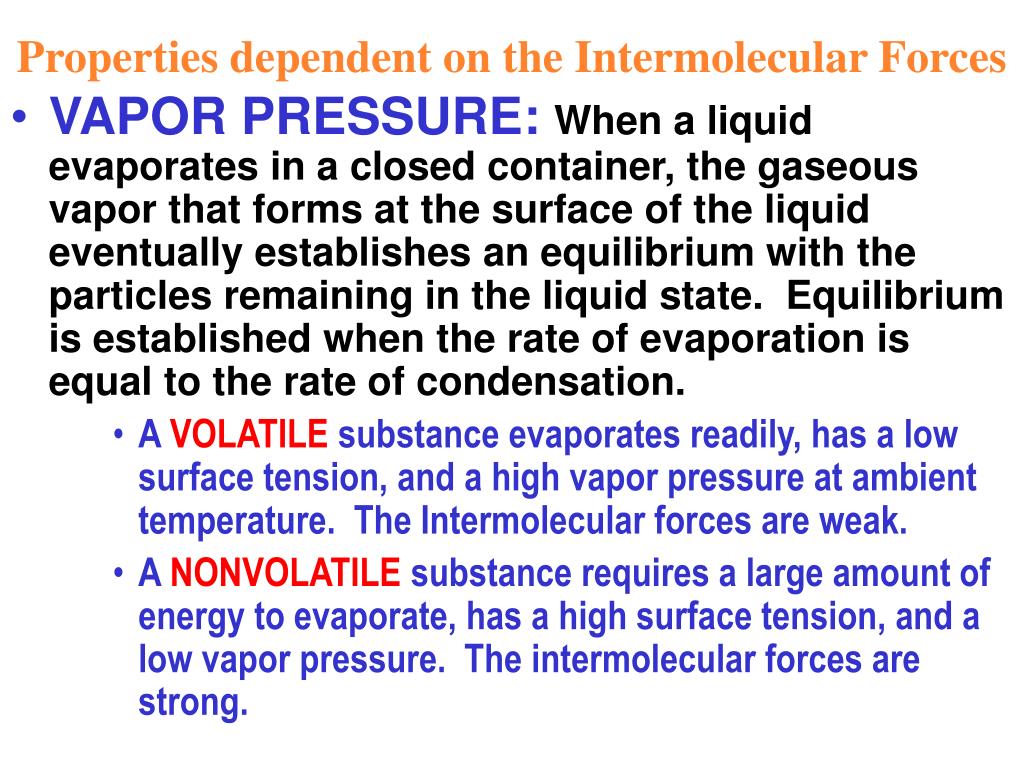
The greater the pressure it exerts, the weaker the intermolecular forces between molecules in its liquid state; the more volatile the liquid; the lower the boiling point and the faster its evaporation rate. Vapor pressure is an indication of a liquid's evaporation rate.
How does external pressure affect the boiling point of a liquid?
Greater the pressure exerted on the liquid, greater would be the boiling point or vice versa. Boiling point of a liquid can be increased by increasing the external pressure on the liquid, whereas it can be decreased by decreasing the external pressure on the liquid. At 1 atmospheric pressure water boils at 100°C.
What happens when vapor pressure is lower than atmospheric pressure?
If the vapor pressure is lower than the atmospheric pressure, it means that the thermal energy of molecules is not strong enough for them in the liquid to break free to enter gas phase. Liquid molecules can not form bubbles of vapor within the liquid. Therefore boiling would never occur.
How does vapor pressure affect boiling point?
Lowering the vapor pressure of a substance has an obvious effect on boiling point; the boiling point goes up. The BP increases because more energy is required for the solvent's vapor pressure to reach the external pressure.
Does low vapor pressure mean strong intermolecular forces?
Substances with strong intermolecular forces have lower vapor pressures and are less volatile, while substances with weak intermolecular forces have higher vapor pressures and are more volatile.
What happens when vapor pressure is less than atmospheric pressure?
If the vapor pressure is lower than the atmospheric pressure, it means that the thermal energy of molecules is not strong enough for them in the liquid to break free to enter gas phase. Liquid molecules can not form bubbles of vapor within the liquid. Therefore boiling would never occur.
What happens if pressure is lower than saturation pressure?
The substance is in the gas (superheated vapor) state if the pressure is less than the saturation pressure at the given temperature or if the temperature is greater than the saturation temperature at the given pressure.
What happens to the volume of a gas in the gas phase?
Let me try to put what happens in this effect into words: This is an effect already present when gasses are assumed to behave ideally. Hence in the gas phase the vapour and the other gas don't interfere in any way. When some volume of the liquid vapourises, the volume available for the inert gas increases slightly. With this volume increase, also the entropy of the gas increases, as the particles have more places where to be, statistically speaking. Furthermore, this increase in entropy is also proportional to particle number, which is proportional to the pressure at constant temperature. Hence the higher the pressure the more liquid will evaporate.
What happens when you increase the pressure of a liquid?
2) If we increase the external pressure on the liquid while temperature is held constant, a higher total pressure on the liquid increases the vapor pressure (from molecules escaping the pure liquid into the gas phase).
What is the difference between P and V?
where P is the total pressure acting on the pure liquid (caused by the combination of the condensible and non-condensible gases in the gas phase) and V is the molar volume of the liquid. So, for the pure liquid,
What is the potential between two atoms making up a compound?
I would rather say that the potential between two atoms making up the compound is approximately parabolic with constant width (you may also assume a van der Waals or 6-12 potential) and compression leads to a deviance of the interatomic distance from the least energy minimum. To formulate it differently, at larger distances than the equilibrium distance the atoms attract, but at lower distances they start to repell each other (Pauli repulsion).
Does adding species B to the gas phase increase the total pressure?
No. If we add as insoluable gas species B to the gas phase, that increases the total pressure. That causes the free energy of the pure liquid A to increase, via the equation I wrote, where V is the molar volume of liquid A (which is much smaller than the molar volume of A vapor). This increase of the free energy of species A in the liquid has to be matched by a corresponding increase in the free energy of A in the gas phase, in order to maintain phase equilibrium. So its equilibrium partial pressure has to change.
Does pressure compress bonds?
Good point. I would say that the pressure compresses the bonds whence they become weaker.
