Lab labels are to be created for the same reasons as workplace labels and require the same information as workplace labels:
- Product identifier (product name matching that on the SDS)
- Information on the safe handling of the product
- A statement that the SDS is available
What are the labeling requirements of the laboratory standard?
Specifically, the labeling requirements of the Laboratory standard are provided in paragraph 1910.1450 (h), which include: 1910.1450 (h) (1) (i) Employers shall ensure that labels on incoming containers of hazardous chemicals are not removed or defaced.
How do you label a specimen for lab work?
Specimen Labeling Requirements. 1. First and last name 2. Date of birth, or social security number 3. Date of collection and time of collection must be written on the requisition 4. Source of specimen if not blood. For example, catherized urine, stool, sputum, right or left synovial fluid 5.
What information should be on a chemical label?
If the chemical is hazardous, the container must include a warning about the respective hazard (eg: eye irritant, skin irritant, etc.) Manufacturer’s name and address must be displayed on the label. Hazard warning must be written in English and include words, pictures, symbols, or a combination of the three.
What are the labeling requirements for secondary containers of hazardous chemicals?
The Laboratory standard requires that labels on incoming containers of hazardous chemicals not be removed or defaced, per paragraph 1910.1450 (h) (1) (i), but does not have a specific labeling requirement for secondary containers of hazardous chemicals in a covered laboratory.
What is the labeling requirement for a laboratory?
What is the definition of laboratory in OSHA?
What is a protective laboratory?
What is a laboratory scale?
Does OSHA require labeling of secondary containers?
See 2 more
About this website
What does a laboratory label usually include?
the product identifier, the chemical name or generic chemical name of the BIM, the initial supplier identifier, and. the statement "Hazardous Laboratory Sample.
Which of the following information is required to be on a label?
All labels are required to have pictograms, a signal word, hazard and precautionary statements, the product identifier, and supplier identification.
What 5 elements must be included within a label?
5 Basic Elements that MUST be on Your Food LabelIngredients.Sugar, fat, and sodium content.Calorie counts and serving size.Freshness.Organic.GMOs.
What are the 3 things required on a workplace label?
In general, a workplace label will require the following information: Product name (matching the SDS product name). Safe handling precautions, may include pictograms or other supplier label information. A reference to the SDS (if available).
What are the six required elements of a product label?
Question: What is the correct grouping/placement of the six required elements for labels on shipping containers (product identifier, signal word, hazard statement, pictogram, precautionary statement, and name/address of the manufacturer), per 1910.1200(f)?
What must be written on every specimen label?
To maintain patient safety standards, specimens must be properly labeled with the name of the patient, a numerical identifier that is unique to the patient (like DOB or SSN), collection date, and the source of the specimen, where applicable.
What are the 4 things that must be on a food label?
Nutrition labels must display the amount of energy (calories and kilojoules) and the amount of fat, saturated fat, carbohydrates, sugars, proteins and salt (all expressed in grams) present in 100g (or 100 ml) of the food.
What is label elements?
The label element represents a label which can be associated to a form control, and is supposed to provide a short description for it. Browsers may link both elements by allowing users to set the focus to the control by clicking on its label.
What are the 5 mandatory requirements in labeling packaged food?
Required Packaging Elements and PlacementStatement of Identity. ... Net Quantity of Contents. ... Ingredient Statement. ... Allergen Declaration. ... Name and Address of the manufacturer, packer, or distributor.
How many items are on a workplace label?
The information requirements for a workplace label are general and employers have some flexibility regarding language and format but it must contain three items: A product identifier identical to that on the SDS for the hazardous product.
Which of the following are not one of the 6 pieces of information required on a supplier label?
The correct answer is C. Company logo are not one of the six pieces of information required on a supplier label. Labels are used to describe the chemicals and products that a company manufactures. Labels can help customers find out more about the chemicals they use in their products.
How many pieces of information are required on a supplier label?
Most supplier labels show six types of information. The written information must be shown in both English and French. Supplier labels may be bilingual (as one label) or available as two labels (one in English, and one in French). The pictogram(s), signal word, and hazard statement(s) must be grouped together.
What is required on a hazmat label?
The shape must be a diamond (square on point) and have dimensions of 3.9 in (100 mm) on each side. Each side must have a solid line inner border 5 mm inside and parallel to edge. The width of the solid line border must be at least 2 mm. The sign must be a rectangle measuring at least 3.9 in.
When duplicate labels are required of a hazmat package where must they be located?
When primary and subsidiary hazard labels are required, they must be displayed next to each other. Placement conforms to this requirement if labels are within 150 mm (6 inches) of one another.
Is it okay for a hazmat label to wrap around an edge of a box?
Ensure all the dangerous goods labels and markings (printed/hand written information on the box) are on one side of the package. The labels must lie flat and not wrap around the edges of the package.
What package marking indicates that a material is a hazardous substance?
Hazard Class Labels are standard hazmat identifiers, designed to meet regulations. Each label has markings that tell what type of hazard is identified on the label. They help identify what type of hazardous material is in a package.
Laboratory Safety Labeling and Transfer Facts of Chemicals
uick Facts Laboratory Safety Labeling and Transfer of Chemicals Permanent Container Labels . Employers must ensure that no worker uses, stores, or . allows any other person to use or store any hazardous
Laboratory Safety Guidance - Occupational Safety and Health Administration
Occupational Safety and Health Act of 1970 “To assure safe and healthful working conditions for working men and women; by authorizing en-forcement of the standards developed under
42 CFR § 493.1242 - LII / Legal Information Institute
LII; Electronic Code of Federal Regulations (e-CFR) Title 42 - Public Health; CHAPTER IV - CENTERS FOR MEDICARE & MEDICAID SERVICES, DEPARTMENT OF HEALTH AND HUMAN SERVICES
Guidelines for Labeling Secondary Containers - UGA
Guidelines for Labeling Secondary Containers . These guidelines specify the procedures endorsed by the UGA IACUC for the proper labeling of secondary (non-manufacturer’s) containers for any materials used in the animal facility, whether for
Effective practices for reducing patient specimen and laboratory ...
Publications . Snyder SR, Favoretto A, Derzon J, Christenson C, Kahn S, Shaw C, Baetz RA, Mass D, Fantz CR, Raab SS, Tanasijevic MJ, and Liebow E. Effectiveness of Barcoding for Reducing Patient Specimen
What information is needed for proper processing of samples?
Additional information needed for proper processing of samples is: time and date of collection and source when appropriate.
Do you have to include date and time of specimen collection on the outside of the specimen?
All samples must include date and time of specimen collection on the outside of the collected specimen. For outside referral samples, full patient name, date of birth and/or social security number must be written on the specimen and the requisition.
Why do we need labels?
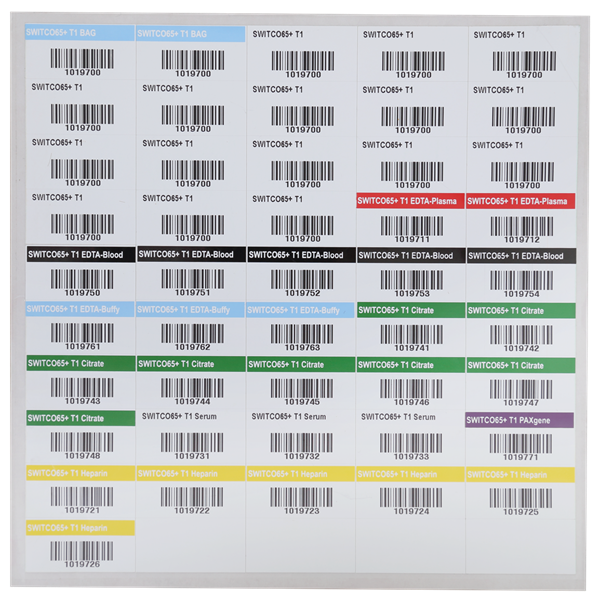
Terrible handwriting isn’t the only reason you may want to switch to a more automated form of labeling. There are a variety of benefits to printed labels, whether you are using an in-house printer, using free online templates, a thermal printing kit, or even a completely automated print-and-apply system. This allows you to fit more information into a small area, and tends to be much more legible than handwriting. Furthermore, the use of specialty ribbons in thermal transfer printing can make the text resistant to strong chemicals and stains, securing your data. It can also allow the addition of colorful graphics such as logos, which can easily identify a specimen and its source (i.e. clinic, CRO, University, etc.). The proper label can make all the difference in ensuring your samples remain well identified.
Why do we need barcodes in labels?
The incorporation of barcodes in your labels can be a quick and error-proof way to manage many samples at once. While some planning and setup are required at the outset, barcoding software is available to automatically serialize your data, saving time in the long-run.
What happens if you mishandle a lab?
We’re all familiar with the devastating consequences that can occur when critical information is mishandled in a labor the clinic: the patient or sample ID is incorrect and the wrong lab result is reported in the clinic, resulting in a patient receiving improper healthcare. This can be compounded by the fact that in clinical studies, a patient’s information and samples are often collected, tested, and analyzed off-site, and sometimes pass through the hands of numerous lab personnel in multiple locations, making it even harder to maintain a corroboration between patient and sample. In rare cases, evidence tampering leads to a wrongful conviction, or sample labeling errors lead to a disastrous mix-up at a fertility clinic! Here are some easy to follow labeling do’s and don’ts to help ensure proper labeling in the lab.
How to reduce the frequency of mislabeled samples?
Measures are now taken more often to reduce the frequency of these errors by creating better data management systems in conjunction with the use of proper labeling techniques. For example, one clinical research facility reduced the frequency of mislabeled or unlabeled samples by an estimated 108 events per year after implementing a barcode-based patient ID system. Labeling errors are also a concern in smaller research labs, where missing specimen information can lead to inefficiency, costing valuable time and sometimes requiring an experiment be redone. Accurate item labeling must be enforced to provide easier communication, organization and a safe working environment.
What is Labtag by GA?
LabTAG by GA International is a leading manufacturer of high-performance specialty labels and a supplier of identification solutions used in research and medical labs as well as healthcare institutions.
What is writing on a tube?
Writing on tiny tubes and small labels is a challenge with a marker or pen. This can complicate communication when other people in the lab need to share samples and reagents, and must fully understand the contents of a tube.
What is a cryo marker?
There are specialty marker pens made specifically for writing on ink-resistant surfaces like plastic, glass and metal . Cryo-markers are also ideal for writing on frozen tubes & vials, with ink that remains legible when exposed to water, frost, and even alcohols.
What is the Laboratory Standard?
The Laboratory standard allows laboratories flexibility in tailoring their written Chemical Hygiene Plan (CHP) and standard operating procedures to be protective of employees in laboratories. See 29 CFR 1910.1450 (e). In addition, 1910.1450 (f) (4), Training, requires the employer to train employees regarding the physical and health hazards ...
What is the OSHA standard for laboratories?
Response: OSHA's Laboratory standard, 29 CFR 1910.1450, covers laboratories meeting the criteria of "laboratory use" and "laboratory scale" but excludes procedures that are part of a production process. See 1910.1450 (b) (definition of "Laboratory use of hazardous chemicals). Research and academic laboratories meeting these criteria are covered by the Laboratory standard and are not covered under the HCS 2012.
What is the HCS 2012 label?
If your facility or a non-laboratory portion of your facility is not covered by the Laboratory standard, the HCS 2012 paragraph 1910.1200 ( f) (6), Workplace labeling, provides employers with flexibility when labeling hazardous chemicals in the workplace. Employers can provide either all of the required information that is on the label from the chemical manufacturer or the product identifier and words, pictures, symbols or a combination thereof, which in combination with other information immediately available to employees, provide specific information regarding the hazards of the chemicals. See 1910.1200 (f) (6) (i) and (ii). In addition, employers are required to train their employees on workplace labeling systems as well as labels received on shipped containers which comply with the HCS 2012 per paragraph 1910.1200 (h) (3) (iv).
What is OSHA interpretation letter?
OSHA requirements are set by statute, standards and regulations. Our interpretation letters explain these requirements and how they apply to particular circumstances, but they cannot create additional employer obligations. This letter constitutes OSHA's interpretation of the requirements discussed. Note that our enforcement guidance may be affected by changes to OSHA rules. Also, from time to time we update our guidance in response to new information. To keep apprised of such developments, you can consult OSHA's website at https://www.osha.gov.
What is OSHA letter?
OSHA requirements are set by statute, standards, and regulations. Our interpretation letters explain these requirements and how they apply to particular circumstances, but they cannot create additional employer obligations. This letter constitutes OSHA's interpretation of the requirements discussed.
How to contact the Office of Health Enforcement?
If you have any further questions, please feel free to contact the Office of Health Enforcement at (202) 693-2190.
Is a company a manufacturer, importer, or distributor of hazardous chemicals?
Scenario: Your company is not a manufacturer, importer, or distributor of hazardous chemicals. Hazardous chemicals are used in your company's lab and facility. The lab you've described supports production as well as research and development. Different sizes of containers of hazardous chemicals are used. The larger 16-ounce bottles of hazardous ...
What is OSHA labeling?
Occupational Safety and Health Administration (OSHA) has a set of labeling guidelines in place for chemicals being used, stored or otherwise handled in laboratories. Whether it’s a medical, clinical, industrial or academic lab, laboratories of all shapes and sizes frequently house a variety of potentially dangerous chemicals.
When is labeling secondary containers necessary?
Labeling secondary containers is necessary when one or more of the following occurs: The worker who made the transfer does not use the chemical in his or her shift. The worker who made the transfer exits the area. The secondary container is moved to a new area.
What are the key points of OSHA?
Key OSHA Points of Lab Chemical Labeling: Chemical must be clearly stated on the label. If the chemical is hazardous, the container must include a warning about the respective hazard (eg: eye irritant, skin irritant, etc.) Manufacturer’s name and address must be displayed on the label.
Do labs need to be monitored?
According to the OSHA’s official website, employers “ must periodically measure employee exposures to harmful substances if you suspect that these exposures are routinely above the action level (i.e., the threshold for increased compliance activities such as air monitoring and medical examinations) .” However, monitoring is only required when the chemical levels are above the “action” level.
Is There an OSHA Standard For Lab Chemicals?
Before we go into the exact labeling requirements for lab chemicals, it’s important to note that the OSHA has a standard — Title 29 of the Code of Federal Regulations (CFR) 1910.1450, Occupational Exposure to Hazardous Chemicals in Laboratories — in place for lab chemicals. Lab managers and employees can refer to this standard to learn more about what’s acceptable and what’s not in a lab environment. (see https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_id=10106&p_table=STANDARDS for more information).
What is the most important part of a nutrition label?
1. Serving Sizes and Servings per Package. Serving sizes and servings per package are arguably the most important part of a nutrition label, since all nutrition fact information is based on the particular serving size.
When can you keep nutrition facts?
There will be some leniency early on, as products packaged on or before December 31, 2019, will be allowed to keep the current Nutrition Facts label until the product is out of date. The Bottom Line. There are a lot of nutrition label requirements—there’s no doubt about that.
How does the FDA protect consumers?
With the help of the FDA’s requirements, you can help protect consumers by encouraging them to make healthy, informed food choices for themselves and their families. Just when you thought you were up to speed on what’s required and what’s optional to disclose on your nutrition label, the FDA goes and adjusts it.
Why is the demand for reliable nutrition information increasing?
More consumers are elevating their health consciousness, particularly with the rising number of concerns about the increasing levels of obesity in our population. Heart disease is the leading cause of death in the United States every year—accounting for 1 in every 4 deaths—so it’s no wonder why Americans are buckling down on the food they’re putting in their bodies. 1
Is vitamin D required on the nutrition label?
Vitamin D, Potassium, and the minerals, calcium and iron, will now state exact amounts along with their daily value percentage. Vitamins A and C will no longer be required on the FDA’s Nutrition Facts labels (though manufacturers may still include them if they choose), while Vitamin D and Potassium will now be required.
Is a declaration of sugar content or category required?
A declaration of sugar content or category is not required if the statement “not a significant source of sugars” is placed at the bottom of the nutrition facts table. Again, if a serving has less than 0.5 grams of sugars per serving, zero may be used. 11. Protein. Protein is an incredibly important nutrient.
Do food companies have to list every nutrient?
Detailed nutrition information is one of the best ways food manufacturers can encourage consumers to make good decisions with regard to their eating habits—but food companies aren’t required to list every nutrient in the food they provide.
What is the federal law for labeling medical devices?
The Federal Food, Drug and Cosmetic Act (FFDCA) is the law under which the FDA takes action against regulated products. Specifically:
What is labeling vs labeling?
Label vs. Labeling. The U.S. Food and Drug Administration (FDA) develops and administers regulations under authority granted by laws passed by Congress that apply to food, drugs, cosmetics, biologics, radiation-emitting electronic products, and medical devices. Labeling regulations pertaining to medical devices are found in the following Parts ...
Does immediate container include package liners?
The term 'immediate container' does not include package liners. Any word, statement, or other information appearing on the immediate container must also appear 'on the outside container or wrapper, if any there be, or the retail package of such article, or is easily legible through the outside container of wrapper.'.
Is advertising labeling?
Advertising. According to an appellate court decision: "Most, if not all advertising, is labeling. The term 'labeling' is defined in the FFDCA as including all printed matter accompanying any article. Congress did not, and we cannot, exclude from the definition printed matter which constitutes advertising.".
What is the labeling requirement for a laboratory?
Specifically, the labeling requirements of the Laboratory standard are provided in paragraph 1910.1450 (h), which include: 1910.1450 (h) (1) (i) Employers shall ensure that labels on incoming containers of hazardous chemicals are not removed or defaced.
What is the definition of laboratory in OSHA?
Response: For a facility to be covered under OSHA's Laboratory standard, 29 CFR 1910.1450, it must satisfy the standard's definition of a laboratory: a facility where the "laboratory use of hazardous chemicals" occurs. This means the handling or use of hazardous chemicals where all of the following conditions are met:
What is a protective laboratory?
2 "Protective laboratory practices and equipment" means those laboratory procedures, practices and equipment accepted by laboratory health and safety experts as effective, or that the employer can show to be effective, in minimizing the potential for employee exposure to hazardous chemicals. 29 CFR 1910.1450 (b).
What is a laboratory scale?
1 "Laboratory scale" means work with substances in which the containers used for reactions, transfers, and other handling of substances are designed to be easily and safety manipulated by one person, excluding workplaces whose function is to produce commercial quantities of materials. 29 CFR 1910.1450 (b).
Does OSHA require labeling of secondary containers?
Unlike the Hazard Communication standard, OSHA's Laboratory standard does not specifically address the labeling of secondary containers in the laboratory. The Laboratory standard allows laboratories flexibility in tailoring their written Chemical Hygiene Plan (CHP) and standard operating procedures to be protective of employees in laboratories (29 CFR 1910.1450 (b)). In addition, paragraph 1910.1450 (f) (4), Training, requires the employer to train employees regarding the physical and health hazards of chemicals in the work area, the measures employees can take to protect themselves from these hazards, and the employer's CHP.
