Full Answer
What are the disadvantages of Bradford protein assay?
Thus, if the protein does not contain an ideal number of aromatic residues, then the dye will not be able to bind to the protein efficiently. Another disadvantage of the Bradford Protein Assay is that this method depends on comparing the absorbance of the protein to that of a standard protein.
What are the standards used in Bradford assay?
The Bradford assay uses standards to both quantify the amount of protein in samples and to subtract any background due to interfering substances that can shift the ratios between the three forms of the dye. The concentration range of standards in the kits cover the linear range of the Bradford assay.
Does detergent interfere with Bradford assay?
Bradford assay does not like detergent. 0.1% SDS should be ok, but NP-40 and sodium deoxycholate likely not, especially with all three present. See The BCA assay is far less affected by detergents (reducing agents will interfere with either of these assays).
What chemicals are compatible with a standard Bradford protein assay kit?
A standard Bradford protein assay kit is compatible with the following chemicals: Denaturing agents such as sodium thiocyanate, guanidine HCl, urea, and phenol Reducing agents such a dithiothreitol and β-mercaptoethanol Tissue culture media such as Eagle's MEM and Hank's salt solution
What can affect Bradford assay?
Factors such as; temperature, wavelength, detergents and even the type of cuvettes you use can influence the measurement and give you wrong results.
What compounds interferes with Bradford assay?
While the Bradford assay is clearly superior to the nitrogen based tests, there are some compounds that interfere with it. It is subject to interference by detergents such as sodium dodecyl sulfate (SDS), Triton X-100 and commercial glassware detergents (Bradford, 1976).
What are the limitations of Bradford assay?
The main limitation of the Bradford assay is its incompatibility with most detergents, routinely used to solubilize membrane proteins. (Interestingly, however, very low levels of non-ionic detergent, such as Triton X-100, may improve sensitivity and variability of the Bradford assay [25] ).
What are the possible reasons for a lower than expected result with the Bradford assay?
Problem: Absorbance of the standard is lower than expectedPossible Cause: Dye reagents are too old or were improperly stored. ... Possible Cause: Standard dilutions prepared incorrectly. ... Possible Cause: Dye reagent may be too cold. ... Possible Cause: Absorbance measured at incorrect wavelength.
Does DNA interfere with Bradford assay?
It was found that Coomassie Blue G-250 in Bradford Assay reagent does interact with DNA at approximately one-fifteenth the rate of the interactions with standard bovine serum albumin.
How does SDS affect Bradford assay?
The standard Bradford protein assay is insensitive to collagen. But if a small, sub-threshold amount of SDS is added to the sample, the response to collagen is increased by at least an order of magnitude, while, on average, the sensitivity for non-collagens is decreased by approximately a factor of 2.
Why is Bradford assay not always accurate?
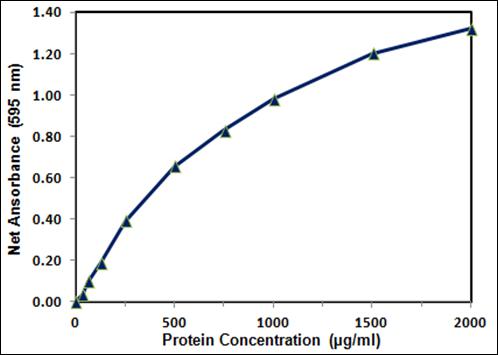
The Bradford assay is linear over a short range, typically from 0 µg/mL to 2000 µg/mL, often making dilutions of a sample necessary before analysis. In making these dilutions, error in one dilution is compounded in further dilutions resulting in a linear relationship that may not always be accurate.
What are the drawbacks of using Bradford's method?
The biggest disadvantage of the Bradford protein assay is that it doesn't work if detergents or surfactants are in the sample, or if the sample is basic. Particularly surfactants that are often used to solubilize some types of proteins will interfere with the test, causing the dye to precipitate out.
How accurate is the Bradford assay?
The Bradford assay is very fast and uses about the same amount of protein as the Lowry assay. It is fairly accurate and samples that are out of range can be retested within minutes.
Why do you need to dilute your protein in Bradford assay?
The protein standard or unknown must be sufficiently diluted not to interfere with the low pH achieved by the acid in the reagent.
Why does the Bradford reagent change colour when protein is added?
The binding of the complex to protein causes a shift in the absorption maximum of the dye-metal complex from 450 to 660 nm. The dye-metal complex has a reddish brown color that changes to green on binding to... The Bradford assay relies on the binding of the dye Coomassie Blue G250 to protein.
Is Bradford reagent light sensitive?
Bradford is also sensitive to various common chemicals in the solution, including detergents and caotropic agents. UV is sensitive to anything that absorbs UV light (nucleic acids, nucleotides, aromatic substances...)
What specific amino acids interact with the Bradford reagent?
Bradford dye can bind to these amino acids in protein sample - arginine (ARG) and phenylalanine (PHE), tryptophan (TRY), and proline (PRO) (aromatic amino acid residues).
What does BCA react with?
BCA is a weak acid composed of two carboxylated quinoline rings and serves the purpose of the Folin reagent in the Lowry assay, namely, to react with the product (Cu1 + ions) of complexes between copper ions and peptide bonds to produce a purple end product that strongly absorbs at 562 nm (Smith et al., 1985).
Why is Bradford assay not always accurate?
The Bradford assay is linear over a short range, typically from 0 µg/mL to 2000 µg/mL, often making dilutions of a sample necessary before analysis. In making these dilutions, error in one dilution is compounded in further dilutions resulting in a linear relationship that may not always be accurate.
Is Bradford reagent light sensitive?
Bradford is also sensitive to various common chemicals in the solution, including detergents and caotropic agents. UV is sensitive to anything that absorbs UV light (nucleic acids, nucleotides, aromatic substances...)
Problem: Absorbance of the protein sample is higher than expected
Possible Cause: The concentration of your protein is too high. Solution: Dilute your protein sample and measure the protein concentration again.
Problem: Absorbance of the standard is lower than expected
Possible Cause: Dye reagents are too old or were improperly stored. The Bradford Reagent has an expiry time of approximately 12 months. If older, replace it with a new one and store it at 4°C.
Problem: Samples are dark blue
Possible Cause: High alkaline concentrations. Solution: This raises the pH above the Bradford reagent’s limit. Dilute or dialyze your sample.
Problem: Precipitates in the sample
Possible Cause: Detergents in your protein buffer. Solution: Dialyze your protein sample or dilute the sample to reduce the level of detergents.
Problem: The protein sample contains interfering substances
One of the major problems encountered when performing a Bradford assay is the presence of interfering substances in the buffer. Table 1 summarizes a list of the most commonly used substances and their compatible concentrations. For detailed information, please refer to the manufacturers’ protocol.
What is Bradford protein assay?
The Bradford protein assay is a time-tested colorimetric assay. When the Bradford reagent (acidified Coomassie Brilliant Blue G-250) binds to proteins, the dye undergoes a color change in the visible spectrum, with the absorbance maximum moving from 470 to 595 nm. The absorbance at 595 nm is then read either in a spectrophotometer or a microplate reader and is directly proportional to the amount of protein bound. The exact protein concentration of the sample is determined by interpolation from a standard curve made by measuring the absorbance of a dilution series of protein standards of known concentrations within the linear response range of the Bradford protein assay. Proteins commonly used as standards include bovine serum albumin (BSA) and bovine γ-globulin (BGG).
How many concentrations of standard are needed for Bradford protein assay?
If necessary, the accuracy of the standards can be increased by preparing the standard dilutions in the same buffer as the samples. Typically, seven concentrations of standards are prepared to cover the linear range of a Bradford protein assay, ranging from 0.125 to 2 mg/ml.
What is the BSA in Bradford?
Bio-Rad Bradford protein assay kits include either of two different proteins, BSA or BGG, as standards to construct a standard curve for the relative quantitation of the proteins in the samples. For most determinations of protein concentration, relative values are generally sufficient. BSA is the most commonly used standard for relative protein concentration determination in most laboratories, although the color response of γ-globulin is usually more representative of true concentration for samples that do not have a high albumin content.
What is the most commonly used standard for relative protein concentration determination in most laboratories?
BSA is the most commonly used standard for relative protein concentration determination in most laboratories, although the color response of γ-globulin is usually more representative of true concentration for samples that do not have a high albumin content.
Do free amino acids interfere with a peptide assay?
Additionally, free amino acids and small peptides (<3 kDa) do not interfere with the assay.
Is Bradford protein assay compatible with protein preparations?
All protein assays are subject to interference by certain substances under some conditions. The Bradford protein assay is quite robust and is compatible with many compounds commonly found in protein preparations. A standard Bradford protein assay kit is compatible with the following chemicals:
How does Bradford assay work?
The basis for the Bradford assay is that in order for the Coomassie dye to bind stably to protein, it needs to be doubly protonated. When the dye comes in contact with protein, the first electron is donated to charged groups on the protein. This disrupts the structure of the protein, resulting in exposure of hydrophobic pockets. The dye binds to these pockets, with the sulfonic acid groups binding to positive amines. In addition, there is attraction due to Van der Waals forces. The stably bound Coomassie G-250 is the blue, unprotonated form.
What is the Bradford assay?
The Bradford assay is based on the use of the dye Coomassie Brilliant Blue G-250, which is frequently abbreviated as Coomassie G-250 or Coomassie Blue. This is one of two Coomassie dyes that are often confused. Coomassie R-250 is used to stain protein gels but is not used in protein assays. In addition to being used in the Bradford assay, Coomassie G-250 can also be used to stain protein gels, although it is less sensitive than Coomassie R-250.
What is the absorbance of Coomassie G 250?
In the absence of protein, when the dye is red, Bradford reagent has an absorbance maximum (Amax) of 470 nm. In the presence of protein, the change to the anionic blue form of the dye shifts the Amaxto 595 nm.
What dye is used for Bradford protein assay?
Choose Bradford protein assays for fast, easy, accurate protein quantitation with Coomassie Blue dye. Ready-to-use reagent and BSA or BGG standard dilutions.
What is the standard for Bradford assay?
The standards used most commonly for the Bradford assay are bovine serum albumin (BSA) and bovine γ-globulin (BGG). Ideally, the standard should be the same proteins in the same ratios as are found in the sample (that is, an absolute reference standard). However, for most samples this is not practical or even possible, and relative rather than absolute concentrations are sufficient in most applications.
Is Coomassie G 250 cationic or anionic?
Under acidic conditions, Coomassie G-250 is cationic, mainly doubly protonated, and is red, whereas in neutral conditions the dye is green, and the anionic form is blue. The Bradford reagent is an acidified solution of Coomassie G-250; the dye is thus primarily protonated and red.
What is Bradford protein assay?
Bradford assays are coomassie dye-binding assays for fast and simple protein quantification. The assay is performed at room temperature and no special equipment is required. Standard and unknown samples are added to preformulated Coomassie blue G-250 assay reagent and the resultant blue color is measured at 595 nm following a short room temperature incubation. Bradford protein assays are compatible with most salts, solvents, buffers, thiols, reducing substances, and metal chelating agents encountered in protein samples.
What is a Pierce compatible Bradford assay?
Pierce Detergent Compatible Bradford Assay Kit is a quick and ready-to-use modification of the well-known Bradford Coomassie dye-binding, colorimetric method for total protein quantitation. This formulation is compatible with up to 1% of commonly used detergents. Comparing Pierce Detergent Compatible Bradford Assay to the Bio-Rad DC Protein Assay, better sensitivity is seen with the Pierce Detergent Compatible Bradford Assay using common detergents. The range of the standard curve for the Pierce Detergent Compatible Bradford assay is 4 times broader than the range for the Bio-Rad DC assay.
What are the disadvantages of Coomassie assays?
The main disadvantage of Coomassie based protein assays is their incompatibility with surfactants at concentrations routinely used to solubilize membrane proteins. In general, the presence of a surfactant in the sample, even at low concentrations, causes precipitation of the reagent. This limitation can be overcome by using Detergent Compatible Bradford Assay. In addition, the Coomassie dye reagent is highly acidic, so proteins with poor acid-solubility cannot be assayed with this reagent. Finally, Coomassie reagents result in about twice as much protein-to-protein variation as copper chelation-based assay reagents.
What is the Pierce 660 nm protein assay?
The Pierce 660nm Protein Assay is based on the binding of a unique dye-metal complex to protein in acidic conditions that causes a shift in the dye's absorption maximum, which is measured at 660 nm. The dye-metal complex is reddish-brown and changes to green upon protein binding. The color change is produced by deprotonation of the dye at low pH facilitated by interactions with positively charged amino acid groups in proteins. Therefore, the dye interacts mainly with basic residues in proteins, such as histidine, arginine and lysine and to a lesser extent tyrosine, tryptophan and phenylalanine.
What is the linear range for BSA?
B: Typical color response curved using the test tube procedure. The linear detection ranges are 25 to 2000µg/mL for bovine serum albumin (BSA) and 50 to 2000µg/mL for bovine gamma globulin (BGG). Due to the inherent protein to protein variability of all protein assays (37% for the 660nm Protein Assay), this demonstrates that appropriate standards should be used for the type of unknown samples being measured.
Does IDCR dissolve in a protein assay?
The IDCR completely dissolves by thorough mixing and does not have any effect on the assay.
Is Coomassie dye acidic?
In addition, the Coomassie dye reagent is highly acidic, so proteins with poor acid-solubility cannot be assayed with this reagent. Finally, Coomassie reagents result in about twice as much protein-to-protein variation as copper chelation-based assay reagents.
How much does reagent exceed sample?
Remember, as long as you add the same amount of reagent to your standards and samples alike, it doesn't matter much what the relative amounts are, although reagent typically exceeds sample by 10 or 20 to 1.
Why does RIPA turn blue?
RIPA turns blue by itself when Bradford is added making it really hard to quantitate the actual protein concentration in the sample.
Is Bradford reagent compatible with RIPA?
Bradford reagent is not compatible with buffers containing detergents. I suggest DC protein assay by Bio-rad to use with RIPA or NP40.
Is NP-40 compatible with RIPA?
1% NP-40 of RIPA could also be a problem , bcz when u look at the compatibility chart of Bradford assay, more than 0.5 NP is non compatible.
Is BCA assay affected by detergents?
The BCA assay is far less affected by detergents (reducing agents will interfere with either of these assays). See
All Answers (2)
"...Bradford method of protein denaturation" I couldn`t really get it what you mean?
Similar questions and discussions
How can we explain differences in Bradford assay and SDS-Page Coomassie staining ?
What is the interference in Bradford protein assay?
Unlike other protein assays, the Bradford protein assay is less susceptible to interference by various chemical compounds such as sodium, potassium or even carbohydrates like sucrose, that may be present in protein samples. An exception of note is elevated concentrations of detergent. Sodium dodecyl sulfate (SDS), a common detergent, may be found in protein extracts because it is used to lyse cells by disrupting the membrane lipid bilayer and to denature proteins for SDS-PAGE. While other detergents interfere with the assay at high concentration, the interference caused by SDS is of two different modes, and each occurs at a different concentration. When SDS concentrations are below critical micelle concentration (known as CMC, 0.00333%W/V to 0.0667%) in a Coomassie dye solution, the detergent tends to bind strongly with the protein, inhibiting the protein binding sites for the dye reagent. This can cause underestimations of protein concentration in solution. When SDS concentrations are above CMC, the detergent associates strongly with the green form of the Coomassie dye, causing the equilibrium to shift, thereby producing more of the blue form. This causes an increase in the absorbance at 595 nm independent of protein presence.
How to do Bradford protein assay?
It is done in one step where the Bradford reagent is added to a test tube along with the sample. After mixing well, the mixture almost immediately changes to a blue color. When the dye binds to the proteins through a process that takes about 2 minutes, a change in the absorption maximum of the dye from 465 nm to 595 nm in acidic solutions occurs. This dye creates strong noncovalent bonds with the proteins, via electrostatic interactions with the amino and carboxyl groups, as well as Van Der Waals interactions. Only the molecules that bind to the proteins in solution exhibit this change in absorption, which eliminates the concern that unbound molecules of the dye might contribute to the experimentally obtained absorption reading. This process is more beneficial since it is less pricey than other methods, easy to use, and has high sensitivity of the dye for protein.
What happens when SDS concentrations are above CMC?
When SDS concentrations are above CMC, the detergent associates strongly with the green form of the Coomassie dye, causing the equilibrium to shift, thereby producing more of the blue form. This causes an increase in the absorbance at 595 nm independent of protein presence.
What happens if a protein does not contain aromatic residues?
Thus, if the protein does not contain an ideal number of aromatic residues, then the dye will not be able to bind to the protein efficiently. Another disadvantage of the Bradford Protein Assay is that this method depends on comparing the absorbance of the protein to that of a standard protein.
What dye is used for Bradford method?
Figure 1. Coomassie brilliant blue G-250, the binding dye for the Bradford Method
What is the highest absorption of protein?
Many protein-containing solutions have the highest absorption at 280 nm in the spectrophotometer, the UV range. This requires spectrophotometers capable of measuring in the UV range, which many cannot. Additionally, the absorption maxima at 280 nm requires that proteins contain aromatic amino acids such as tyrosine (Y), phenylalanine (F) and/or tryptophan (W). Not all proteins contain these amino acids, a fact which will skew the concentration measurements. If nucleic acids are present in the sample, they would also absorb light at 280 nm, skewing the results further. By using the Bradford protein assay, one can avoid all of these complications by simply mixing the protein samples with the Coomassie brilliant blue G-250 dye (Bradford reagent) and measuring their absorbances at 595 nm, which is in the visible range.
What is the absorption spectrum of cationic dye?
The cationic (unbound) form is green / red and has an absorption spectrum maximum historically held to be at 465 nm. The anionic bound form of the dye which is held together by hydrophobic and ionic interactions, has an absorption spectrum maximum historically held to be at 595 nm. The increase of absorbance at 595 nm is proportional to the amount of bound dye, and thus to the amount (concentration) of protein present in the sample.
