
What drives bacteria to produce a biofilm?
four potential incentives behind the formation of biofilms by bacteria during infection are considered: (1) protection from harmful conditions in the host (defense), (2) sequestration to a...
Why are biofilms important Quizlet?
the biofilm provides a stable environment and nutrients for bacterial cells to proliferate in flowing systems Why do biofilms exist? protection from predatory organisms, antimicrobial agents and host immune system; nutrient supply Quorum sensing bacteria's ability to communicate via secreted chemical signals Majority of microorganisms are found
Why do bacteria form biofilms?
four potential incentives behind the formation of biofilms by bacteria during infection are considered: (1) protection from harmful conditions in the host (defense), (2) sequestration to a nutrient-rich area (colonization), (3) utilization of cooperative benefits (community), (4) biofilms normally grow as biofilms and planktonic cultures are an …
What are examples of biofilms?
Biofilms are a collective of one or more types of microorganisms that can grow on many different surfaces. Microorganisms that form biofilms include bacteria, fungi and protists. One common example of a biofilm dental plaque, a slimy buildup of bacteria that forms on the surfaces of teeth. Pond scum is another example.
What are the microorganisms that make biofilm?
The microorganisms that form biofilms include bacteria, fungi, and protists. Perhaps the most common biofilm familiar to most is the dental plaque — that sticky, colorless film of bacteria and sugars that constantly forms on our teeth. That slime on the surface of water, particularly ponds, is also biofilm.
How long have biofilms been around?
In fact, biofilms have been around for at least 3.3 billion years. However, it’s in wet and moist environments that you’ll the most biofilms. They love wetness. ADVERTISEMENT. A vast number of pathogens are grouped as biofilms.
How big can biofilm get?
How big can a biofilm get. Most biofilms are very thin — just a few cell layers thick. That’s too thin to see with the naked eye. In fact, your kitchen counter almost certainly has a biofilm layer on it. You just can’t see it. Some biofilms, however, can grow many inches thick and are obviously noticeable.
Why are biofilms important?
Biofilms have been poorly understudied until recently but evidence suggests they’re involved in many human diseases , including debilitating chronic infections. According to Dr. Trevor Marshall, a biomedical researcher at Murdoch University, Australia, some large microbiota of chronic biofilm like L-shaped bacteria can evade the immune system because, long ago, they evolved the ability to reside inside macrophages. Ironically, these are the very white blood cells of the immune system which are supposed to kill the invading pathogens. Marshall also says that biofilm infections occur with great ease in immunocompromised hosts.
What is the biofilm that causes endocarditis?
This type of biofilm is known as a vegetation. The vegetation can disrupt valve function, produce a near-continuous infection of the bloodstream, and can block blood circulation through a process known as embolization.
Why do bacteria band together?
As mentioned, bacteria band together because as a community they enhance their chance of survival, but what threats do they face and how does living a slime mold protect them? Some of the stressors bacteria face are the lack of water, high or low pH, or the presence of ‘toxic’ substances, i.e. antibiotics or antimicrobials.
Do antibiotics kill biofilm?
After high doses of antibiotics are administered, it may seem the biofilm infection has disappeared. However, it will reappear because the biofilm was not destroyed, only weakened. It seems that while antibiotics can penetrate the biofilm matrix and kill bacteria, a number of cells called ‘persisters’ are left behind.
How does biofilm spread?
Detachment/Dispersal: Biofilm spreads by detaching small or large clumps of cells, or by a type of “seeding dispersal” that releases individual cells allowing the bacteria to attach to a surface or biofilm downstream. Think of the release of seeds from a dandelion, this is a similar process of encouraging species growth.
What are the stages of biofilm formation?
Biofilm formation commonly occurs in three main stages: Attachment: Single free-floating bacteria land on surfaces and bacterial cells aggregate and attach to one another. Pathogens know they have the best chance of survival as part of a community, or biofilm.
Why is biofilm important?
Biofilm protects pathogens from disinfection and allows organisms injured by environmental stress and disinfectants to recover and grow, which can lead to improved resistance to antimicrobials and antibiotics over time as ‘stronger’ organisms continue to survive. It is imperative to completely remove biofilm to avoid continued resistance.
Why do biofilms form in stagnant water?
Biofilms form more readily in warm, stagnant water. Water flow, or the stress from the flow of water, is an important factor that can slow the growth of biofilm. Additionally, organism type can impact biofilm production. Some organisms produce large amounts of EPS and therefore grow a thicker biofilm.
How to protect against biofilm?
Protection against biofilm. The best way to protect against biofilm is prevent its formation. Simply killing free-swimming and surface level biofilm-related microorganisms, without removing the biofilm structure from a surface, can result in rapid recolonization of organisms from inside the remaining biofilm matrix.
How much does biofilm cost?
Biofilm. Many of us have heard of it but struggle to accurately define and understand. Biofilms cost the U.S. billions of dollars every year in energy losses, equipment damage, product contamination and medical infections.
Why are biofilms considered a public health threat?
Biofilms are considered a public health threat because of their outstanding resistance to antibacterial treatments and disinfection. Biofilm is a grouping of cells that stick together, embedded in a self-produced matrix of extracellular polymeric substance (EPS) composed of proteins, polysaccharides and other materials.
How Are Biofilms Formed?
Biofilm formation begins with planktonic, or free-swimming, bacteria, which land on a surface. Bacteria can attach to a variety of surfaces, from woods, metals, and plastics to living tissues and stagnant water. The cells are able to attach to the surface by excreting a sugary molecule that holds the cells together and attaches them to the surface. This sugary substance is called extracellular polymeric substance, or EPS, and has a strand-like structure that allows it to bind to the surface and to other cells, creating a matrix.
Why are biofilms important?
Research has shown that the formation of biofilms can make bacteria less susceptible to antimicrobial products. The thick, slimy layer can protect bacteria in the middle of the biofilm from the effects of an antimicrobial. This is especially concerning where human health may be affected (for example, on medical devices or in food handling areas). The survival power of bacteria in a biofilm is much higher than that of planktonic bacteria.
What is the purpose of the slimy layer in biofilm?
The thick, slimy layer can protect bacteria in the middle of the biofilm from the effects of an antimicrobial. This is especially concerning where human health may be affected (for example, on medical devices or in food handling areas).
What is the survival power of biofilms?
The survival power of bacteria in a biofilm is much higher than that of planktonic bacteria. While slimy bacterial growth may seem like a negative, there are some surprising benefits of biofilms. On the plus side, biofilms can be used to filter and clean wastewater.
What is the biofilm in the large intestine?
Researchers have also discovered that our large intestines support a biofilm of beneficial bacteria that help us digest our food and may help us form a healthy immune system. Lesson Summary. A biofilm can be made up of many different species of bacteria and may even include algae and fungi.
How does biofilm affect humans?
Biofilms that Affect Humans. Biofilms can take a variety of forms - from the plaque on your teeth to slime buildup in your sink. These microscopic organisms can cause billions of dollars of damage each year in industrial, medical, and domestic settings by clogging equipment and harboring infectious bacteria.
How thick is a biofilm?
A biofilm can be as thin as a single cell or as thick as several inches, depending on conditions in the environment. As a biofilm grows and develops, it thickens and becomes mature. If there is sufficient water and nutrients, the biofilm will develop until small portions detach and float to another surface and colonize.
What is biofilm in the background of many diseases?
Biofilms in the Background of Many Diseases. The medical community is increasingly dealing with antibacterial-resistant infections, with evidence of a biofilm at work behind the scenes: Up to one-third of patients with strep throat, often caused by pyogenes, do not respond to antibiotics ( 9 ).
Where are biofilms found?
Biofilms found along the epithelial lining of the nasal passageways and GI tract are less understood.
Why are biofilms so difficult to detect?
A number of problems make biofilms difficult to detect. First, bacteria within the biofilm are tucked away in the matrix. Therefore, swabs and cultures often show up negative. Stool samples usually do not contain the biofilm bacteria, either. Second, biofilm samples within the GI tract are difficult to obtain.
How to diagnose biofilm?
Biofilms Are Difficult to Diagnose 1 First, bacteria within the biofilm are tucked away in the matrix. Therefore, swabs and cultures often show up negative. Stool samples usually do not contain the biofilm bacteria, either. 2 Second, biofilm samples within the GI tract are difficult to obtain. The procedure would require an invasive endoscope and foreknowledge of where the biofilm is located. What’s more, no current procedure to remove biofilm from the lining of the GI tract exists. 3 Third, biofilm bacteria are not easily cultured. Therefore, even if you are able to obtain a sample, it may again test negative because of the microbes’ adapted lower nutrient requirements, rendering normal culture techniques null ( 7 ). 4 Fourth, biofilms might also play a role in the healthy gut, making it difficult to distinguish between pathogenic and healthy communities ( 4, 7 ).
Why is biofilm a hostile community?
Since it requires less oxygen and fewer nutrients and alters the pH at the core, the biofilm is a hostile community for most antibiotics. In addition, the biofilm forms a physical barrier that keeps most immune cells from detecting the pathogenic bacteria ( 1, 2 ).
Can biofilm bacteria be cultured?
Third, biofilm bacteria are not easily cultured. Therefore, even if you are able to obtain a sample, it may again test negative because of the microbes’ adapted lower nutrient requirements, rendering normal culture techniques null ( 7 ).
Can antibiotics help with biofilms?
Treating illnesses associated with biofilms using antibiotics is an uphill battle. For example, in patients suffering from IBD, antibiotics appear initially to work, only to be followed by a “rebound,” where the symptoms again flare up, presumably due to bacteria evading the antibiotic within a biofilm ( 4 ).
How does biofilm form?
Biofilm formation begins when free-floating microorganisms such as bacteria come in contact with an appropriate surface and begin to put down roots, so to speak. This first step of attachment occurs when the microorganisms produce a gooey substance known as an extracellular polymeric substance (EPS), according to the Center for Biofilm Engineering at Montana State University. An EPS is a network of sugars, proteins and nucleic acids (such as DNA). It enables the microorganisms in a biofilm to stick together.
What are biofilms?
As research has progressed over the years, biofilms — bacterial and fungal — have been implicated in a variety of health conditions. In a 2002 call for grant applications, the National Institutes of Health (NIH) noted that biofilms accounted "for over 80 percent of microbial infections in the body."
Why are microbes living in biofilms?
For microorganisms, living as a part of a biofilm comes with certain advantages. "Communities of microbes are usually more resilient to stress," Gerlach told Live Science. Potential stressors include the lack of water, high or low pH, or the presence of substances toxic to microorganisms such as antibiotics, antimicrobials or heavy metals.
What are some examples of biofilms?
Microorganisms that form biofilms include bacteria, fungi and protists . One common example of a biofilm dental plaque, a slimy buildup of bacteria that forms on the surfaces of teeth. Pond scum is another example. Biofilms have been found growing on minerals and metals. They have been found underwater, underground and above the ground.
How long have biofilms been around?
Biofilms thrive upon moist or wet surfaces. Biofilms have established themselves in such environments for a very long time. Fossil evidence of biofilms dates to about 3.25 billion years ago, according to a 2004 article published in the journal Nature Reviews Microbiology.
Why are biofilms hard?
For example, the slimy EPS covering can act as a protective barrier. It can help prevent dehydration or act as a shield against ultraviolet (UV) light. Also, harmful substances such as antimicrobials, bleach or metals are either bound or neutralized when they come into contact with the EPS. Thus, they are diluted to concentrations that aren't lethal well before they can reach various cells deep in the biofilm, according to a 2004 article in Nature Reviews Microbiology.
Where can biofilms be found?
Biofilms have been found growing on minerals and metals. They have been found underwater, underground and above the ground. They can grow on plant tissues and animal tissues, and on implanted medical devices such as catheters and pacemakers. Each of these distinct surfaces has a common defining feature: they are wet.
How does biofilm form?
Biofilm formation begins with the adhesion of bacteria onto the electrode surface, followed by the coadhesion and proliferation of microbial cells by the formation of multilayer cell clusters.
What is the process of biofilm formation?
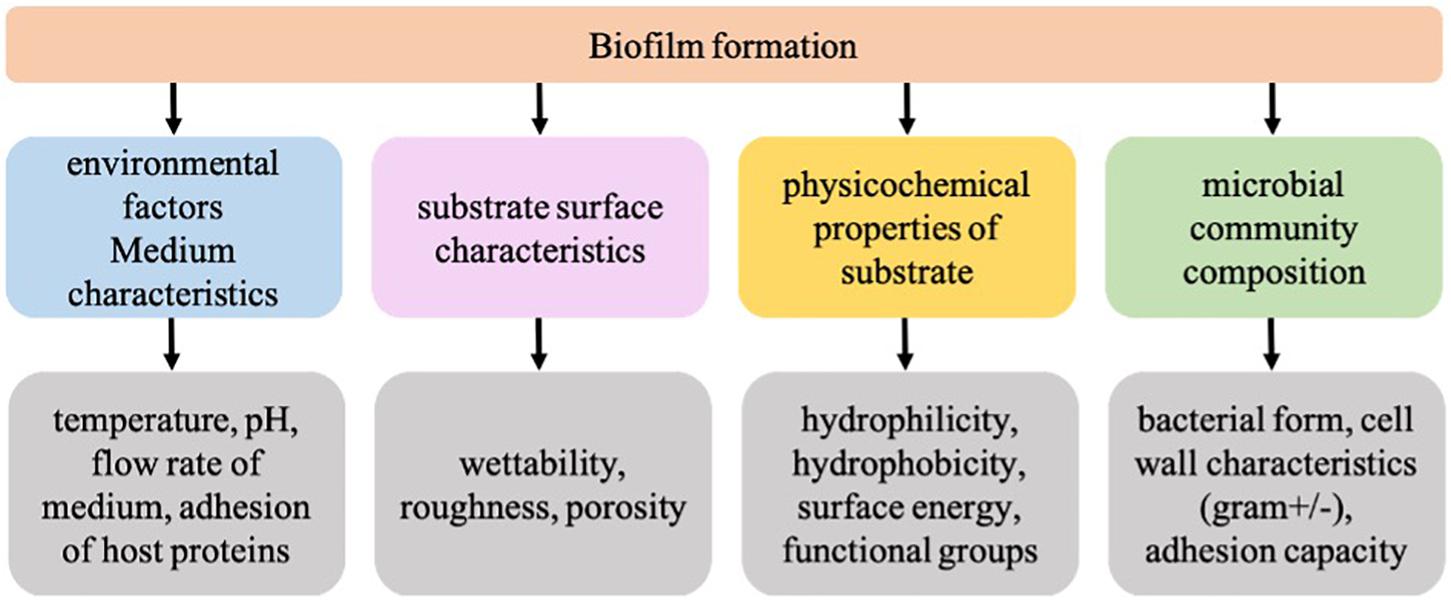
Biofilm formation is a complex process and it involves adhesion, growth, proliferation, inhibition, cell–cell communication, and so on. Reports have shown that more than 100 genes/proteins are involved in biofilm formation [115].
How does Molecular Biology help in biofilm formation?
Molecular biology has been realized as a promising tool for elucidating the mechanisms of biofilm formation, identifying the key molecular factors , and engineering biofilm structure and properties. However, most of the approaches that were reported in the literature were based on the reductionist approach. System biology approaches seem to be a viable option for improving the biofilm characteristics to engineer biofilms for bioelectrochemical applications [113,114]. System biology aims to study the integrated mechanisms by a detailed investigation of the behavior of complex biological organization and biochemical processes using molecular, mathematical, and computational techniques. It has several advantages over the conventional reductionist approach. The reductionist approach aims to study the expression of a single protein or a gene. However, in a system biology approach, different genes/proteins and their interactions are analyzed. Biofilm formation is a complex process and it involves adhesion, growth, proliferation, inhibition, cell–cell communication, and so on. Reports have shown that more than 100 genes/proteins are involved in biofilm formation [115].
What is biofilm in orthopedic surgery?
In this chapter, we review the etiology of infection in orthopedic devices with a focus on the Gram-positive staphylococci as the most commonly isolated pathogens in periprosthetic infections ( Aggarwal et al., 2014 ). This chapter describes the mechanism whereby these bacteria form biofilms in the synovial environment, regulate virulence factors, evade detection, and resist antibiotic action in vivo. Due to the challenges associated with bacteria in the synovial environment, surgical treatments are preferred for the treatment of many joint infections. However, new therapies that address the difficulties of treating infection in synovial fluid could greatly improve outcomes of orthopedic infections.
What are the genes that are involved in biofilm formation?
and lipopolysaccharide biosynthesis proteins, have been shown to play crucial roles in biofilm formation. It is well known that the release of extracellular polymeric substances, such as proteins, polysaccharides, lipids or extracellular DNA is important in the formation of biofilms. Investigations have clearly shown that the deletion of any of these factors greatly affects the community architecture of the biofilms. Type IV pili have been shown to play a key role in the initial surface attachment and in mediating tight cell–cell interactions. The flagella and exopolysaccharides aid in controlling biofilm architecture. c-di-GMP plays a critical role in biofilm formation and stability [107].
How do exoelectrogens contribute to biofilm formation?
The surface characteristics of exoelectrogens make a crucial contribution to enhanced biofilm formation. The greater the surface area of the microorganisms, the more the proteins that are involved in biofilm formation and electron transfer. Several reports are available on correlating the genes coding for conducting proteins and their expression using genomic and proteomic techniques, respectively [110,111]. However, it is also important to correlate the morphology with the proteomic and genomic data. Reports are available on the effect of electron acceptors on the morphometric parameters such as surface area and roughness profile. Dietrich et al. [112] made detailed investigations in Pseudomonas aeruginosa and demonstrated that wrinkling is a redox-driven adaptation that maximizes oxygen accessibility. They established the relationships between the genes and the morphometric parameters in a redox driven electrochemical environment, which will aid in improved biofilm formation and electrocatalysis.
Why is biofilm important?
Biofilm formation is an important survival strategy that bacteria utilize in natural and human-made niches. The detection of adherence of pathogenic bacteria on medical surfaces is necessary to identify and prevent systemic infections related to biofilm-forming bacteria.
What are the biofilms of bacteria?
The ability to form biofilms is a universal attribute of bacteria. Biofilms are multicellular communities held together by a self-produced extracellular matrix. The mechanisms that different bacteria employ to form biofilms vary, frequently depending on environmental conditions and specific strain attributes. In this review, we emphasize four well-studied model systems to give an overview of how several organisms form biofilms: Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, and Staphylococcus aureus. Using these bacteria as examples, we discuss the key features of biofilms as well as mechanisms by which extracellular signals trigger biofilm formation.
What bacteria produces biofilm?
The soil-dwelling Gram-positive bacterium B. subtilisis also studied as a model organism for biofilm formation. Different B. subtil isstrains are able to secrete two distinct polymers: the polysaccharide EPS and poly-δ-glutamate (PGA). Both of these molecules have been described to participate in the process of biofilm formation (Stanley and Lazazzera 2005; Branda et al. 2006). Yet, they contribute differently depending on the strain and conditions studied. For example, in colony biofilms the undomesticated strain NCIB3610 requires exopolysaccharide EPS for biofilm formation (Fig. 1). However, no colony biofilm defect is observed in a mutant strain lacking the ability to produce PGA (Branda et al. 2006). Instead, cells that overproduced PGA formed structureless, mucoid colonies. Another undomesticated strain of B. subtilis, RO-FF-1 naturally produces PGA and forms mucoid colonies. PGA production is important for surface-adhered biofilm formation in both RO-FF-1 and the laboratory strain JH642 (Stanley and Lazazzera 2005). In contrast, the strain NCIB3610 is unable to form robust surface-adhered biofilms (Branda et al. 2006).
What is the biofilm of S. aureus?
Another bacterial model used to study biofilm formation is the Gram-positive pathogen S. aureus. Most strains of S. aureususe a polymer of N-acetyl glucosamine (PNAG) also referred to as polysaccharide intercellular adhesin (PIA), to form biofilms (O’Gara 2007). The icaoperon encodes the machinery that synthesizes this polymer, yet not all strains carry this operon. Even in some of those strains that carry the icaoperon, deletion of the operon does not impair their ability to make biofilm via an ica-independent pathway (O’Gara 2007; Otto 2008). This alternative mechanism relies on the ability of S. aureusto express a variety of adhesin proteins that allow cells to attach and colonize a large number of different surfaces (Lasa and Penades 2006).
What are the molecular mechanisms that regulate biofilm formation?
The molecular mechanisms that regulate biofilm formation vary greatly among different species, and even vary between different strains of the same species. However, some features are recognized as general attributes of biofilm formation (Monds and O’Toole 2009). For instance, all biofilms contain an extracellular matrix that holds cells together. This matrix is often composed of a polysaccharide biopolymer along with other components such as proteins or DNA (Branda et al. 2005). The nature of the matrix exopolysaccharide greatly varies depending on growth conditions, medium, and substrates.
How do biofilms affect humans?
2005; Hall-Stoodley and Stoodley 2009). Biofilms impact humans in many ways as they can form in natural, medical, and industrial settings. For instance, formation of biofilms on medical devices, such as catheters or implants often results in difficult-to-treat chronic infections (Hall-Stoodley et al. 2004; Donlan 2008; Hatt and Rather 2008). Moreover, infections have been associated with biofilm formation on human surfaces such as teeth, skin, and the urinary tract (Hatt and Rather 2008). However, biofilms on human surfaces are not always detrimental. For example, dental plaque biofilms comprise dozens of species and the community composition frequently determines the presence or absence of disease. In dental plaque, there is a progression of colonization and the presence of beneficial species antagonizes colonization by detrimental organisms (Kreth et al. 2008). But biofilms form everywhere. For example, biofilms form on the hulls of ships and inside pipes where they cause severe problems (de Carvalho 2007). On the other hand, in many natural settings, biofilm formation often allows mutualistic symbioses. For instance, Actinobacteriaoften grow on ants, allowing the ants to maintain pathogen-free fungal gardens (Currie 2001; Danhorn and Fuqua 2007). Given the vast potential benefits and detriments that biofilms can confer, it is essential that we understand how bacteria thrive in these communities.
Why are biofilms important for bacteria?
Biofilms confer resistance to many antimicrobials, protection from protozoan grazing, and protection against host defenses (Mah and O’Toole 2001; Matz and Kjelleberg 2005; Anderson and O’Toole 2008). One possible reason for the increased resistance to environmental stresses observed in biofilm cells appears to be the increase in the portion of persister cells within the biofilm (Lewis 2005). Despite being genetically identical to the rest of the population, persister cells are resistant to many antibiotics and are nondividing. Persister cells have been proposed to be protected from the action of antibiotics because they express toxin–antitoxin systems where the target of the antibiotics is blocked by the toxin modules (Lewis 2005). In addition to an increase in persisters, the presence of an extracellular matrix protects constituent cells from external aggressions. Extracellular matrices also act as a diffusion barrier to small molecules (Anderson and O’Toole 2008; Hall-Stoodley and Stoodley 2009). Related to this, in biofilms the diffusion of nutrients, vitamins, or cofactors is slower resulting in a bacterial community in which some of cells are metabolically inactive. Furthermore, the rate of bacterial growth is influenced by the fact that cells within a biofilm are confined to a limited space (Stewart and Franklin 2008). This condition is similar to the stationary phase created in laboratory conditions. Hence, biofilm formation in a way represents the natural stationary phase of bacterial growth. During stationary phase, bacteria profoundly change their physiology by increasing production of secondary metabolites such as antibiotics, pigments, and other small-molecules (Martin and Liras 1989). These secondary metabolites also function as signaling molecules to initiate the process of biofilm formation or to inhibit biofilm formation by other organisms that inhabit the same habitat (Lopez and Kolter 2009). In this article, we review the metabolic processes that characterize biofilm formation for a handful of well-studied bacterial organisms: Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus,and Bacillus subtilis. In addition, we address the function of secondary metabolites and their role as signaling molecules during biofilm formation.
What are the three exopolysaccharides that make up the biofilm?
P. aeruginosais a gram-negative pathogen that makes biofilms by producing three distinct exopolysaccharides: alginate, PEL , and PSL. The importance and contribution of each exopolysaccharide to the matrix varies depending on the strain studied (Ryder et al. 2007; Tart and Wozniak 2008). For example, alginate is a produced by mucoid strains of P. aeruginosathat are often isolated from lungs of cystic fibrosis patients. The pelgene cluster, encoding a glucose-rich polymer termed PEL, is found in most of the strains analyzed to date, yet its expression strongly varies among strains (Branda et al. 2005). The reference strain PA14 used in many laboratories harbors a partial deletion of the psllocus, which prevents the PSL mannose-rich polysaccharide from being made (Friedman and Kolter 2004).
What Are Biofilms and How Are They Formed?
First, we need to define biofilms. Biofilms are colonies of microorganisms—including some of the most common foodborne pathogens—that form on a surface.
How to Identify Biofilms
Biofilms often cannot be seen by the naked eye, so you must take a proactive approach to remove them before they spread. Sensory tests—including visual inspection, the presence of odors, and different textures—can indicate biofilm formation but should not be the only detection method.
How to Treat and Avoid Biofilms
Proactive prevention is always a better approach than reacting to biofilm formation. During daily sanitation, pay special attention to areas where biofilms are more likely to grow. By removing the free-floating bacteria that can potentially form into a biofilm, you can avoid a larger problem in the future.
D7 for Biofilm Treatment
D7 is a patented sanitizer and disinfectant that is EPA-approved for biofilm removal in food processing plants and animal drinking water lines.
