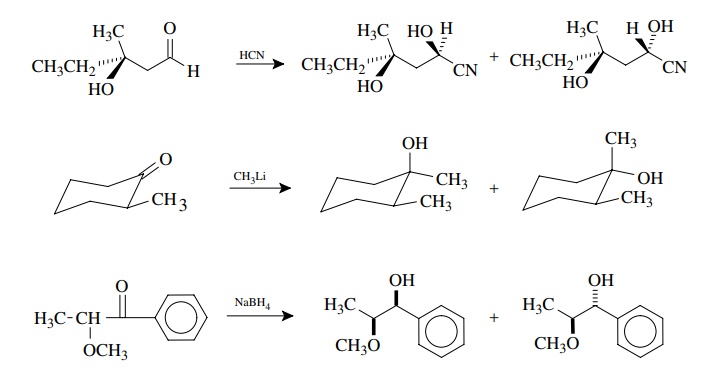
What are Diastereomers Diastereomers (sometimes called diastereoisomers) are a type of a stereoisomer. Diastereomerism occurs when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other. When two diastereoisomers differ from each other at only one stereocenter they are epimers.Diastereomer
Full Answer
Are diastereomers enantiomers?
When it comes to diastereomers, those are, well—not enantiomers. I mean, seriously, the “common” definition of a diastereomers is the stereoisomers that are not enantiomers. The official definition though is the diastereomers are non-superimposable molecules that are not mirror images of each other.
What are chiral centers and diastereomers?
We have two chiral centers, and only one is changed, while the other one is the same. So, these molecules are not mirror images (in mirror images, everything is reflected, therefore changed). And this is what we call Diastereomers: Diastereomers are stereoisomers that are not mirror images of each other.
What is the difference between stereoisomers and diastereomers?
Differences between diastereomers can be expressed in scalar terms, that is by differences in the distances of certain characteristic pairs of atoms. Stereoisomers that are not related as an object and its mirror image are called diastereomers; Diastereomers are stereoisomers that are not mirror images.
Why are diastereomers not mirror images of each other?
They are also not superimposable in space no matter how much you rotate those in space, so they are not the same molecule either. Thus, by definition, they are diastereomers as they are non-superimposable not mirror images of each other.
What is conformational diastereomer?
Diastereomers are defined as non-mirror image, non-identical stereoisomers. Hence, they occur when two or more stereoisomers of a compound have different configurations at one or more (but not all) of the equivalent (related) stereocenters and are not mirror images of each other.
What are conformational stereoisomers?
Conformational isomers (or conformers or rotational isomers or rotamers) are stereoisomers produced by rotation (twisting) about σ bonds, and are often rapidly interconverting at room temperature.
Are diastereomers conformational isomers?
Another class is configurational isomers, which can be separated from one another, as interconversion requires breaking of bonds. There are two types of configurational isomers: diastereisomers and enantiomers. Enantiomers are non-superposable mirror images.
What are the different types of diastereomers?
Other examples of “diastereomers” include: double bond isomers (E/Z) cis–trans isomers [see: cis and trans isomers of cycloalkanes] stereoisomers of molecules with multiple chiral centers that have the same configuration at (at least) one carbon.
How do you identify conformational isomers?
1:302:52Conformational Isomers in Organic Chemistry - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd also sis to each other and if you remember these structures are just the ring flip version ofMoreAnd also sis to each other and if you remember these structures are just the ring flip version of each other. So ring flip versions are conformational isomers.
What is the difference between constitutional and conformational isomers?
The main difference between configurational and conformational isomers is that configurational isomers cannot be obtained by rotating the molecule around a single bond whereas conformational isomers can be obtained by rotating the molecule around a single bond.
What is difference between conformation and configuration?
Conformation is the distinct arrangement of atoms in any molecule which can readily interconvert. Configuration is the distinct arrangement of atoms in any molecule which cannot readily interconvert.
What is the difference between an enantiomer and a diastereomer?
Enantiomers are a pair of molecules that exist in two forms that are mirror images of one another but cannot be superimposed one upon the other. Diastereomers are defined as compounds with the same molecular formula and sequence of bonded elements but are non-superimposable non-mirror images.
Which diastereomer is most stable?
Reasoning based on steric effects is relatively intuitive and gives rise to a generally accepted rule of thumb that an E configuration, anti conformer and para isomer in diastereomers, conformational and constitutional isomers, respectively, should be the most stable forms.
How do you identify a diastereomer?
Molecules that are mirror images but non-superimposable are enantiomers. If they aren't superimposable, and they aren't mirror images, then they're diastereomers.
What is an example of diastereomer?
What are diastereomers with examples? Diastereomers are the stereoisomers that are non identical, do not have mirror images, and hence are non-superimposable on each other. Examples of diastereomers include cis and trans-2-butene, D-threose and D-erythrose, 2-chloro,3-bromobutane, and so on.
What are the 3 types of stereoisomers?
Generally defined, stereoisomers are isomers that have the same composition (that is, the same parts) but that differ in the orientation of those parts in space. There are two kinds of stereoisomers: enantiomers and diastereomers.
What is conformational isomerism with example?
For example, butane has three conformers relating to its two methyl (CH3) groups: two gauche conformers, which have the methyls ±60° apart and are enantiomeric, and an anti conformer, where the four carbon centres are coplanar and the substituents are 180° apart (refer to free energy diagram of butane).
What is conformation in stereochemistry?
Due to this rotation, different spatial arrangements of carbon atoms in space are observed which can change into one another. Such spatial arrangement of carbon and hydrogen atoms which can be converted into one another by rotation around a C-C single bond is called conformation or conformer or rotamer.
What is the difference between conformational isomers and geometrical isomers?
1 Answer. One conformational isomer changes into other without any bond rearrangement i.e. due to single bond rotation while geometrical isomers are converted in to one form to other by bond rearrangement i.e. bond breaking and bond making.
What are conformations in chemistry?
conformation, any one of the infinite number of possible spatial arrangements of atoms in a molecule that result from rotation of its constituent groups of atoms about single bonds.
What is the difference between an enantiomer and a diastereomer?
Enantiomers and diastereomers are two types of stereoisomers. Enantiomers include mirror images and non-superimposable chiral centers. Diastereomer...
What are diastereomers as an example?
Diastereomers may often include compounds which are ring structures. Imagine, for example, two compounds with a six-membered ring, each with two su...
Are enantiomers optically active?
Yes. All enantiomers are involved with optical effects. Optical behaviour is also one of the concepts of enantiomers because enantiomers are molecu...
Are diastereomers always chiral?
Diastereomers are often chiral and distinct from each other. Remember that pairs of diastereomers exist, and each has two chiral centres. The chira...
Which are two types of stereoisomers?
Diastereomerism (including ‘cis-trans isomerism’) Optical Isomerism (also known as ‘enantiomerism’ and ‘chirality’) are the two primary forms of st...
Are D and L enantiomers?
The enantiomers D and L refer to the molecule’s configurational stereochemistry. L isomers have the hydroxyl group positioned on the left side of t...
How do you identify diastereomers?
If there is more than one chiral center in a molecule, you have the risk of making stereoisomers not mirroring each other’s images. These stereoiso...
What are diastereomers with examples?
Diastereomers are the stereoisomers that are non identical, do not have mirror images, and hence are non-superimposable on each other. Examples of...
What is the difference between an enantiomer and diastereomer?
Enantiomers are non-superimposable mirror images of each other, whereas diastereomers are non-superimposable non-mirror images of each other. Any s...
What is stereochemical relationship?
Between two compounds that are stereochemical, there can exist only an enantiomeric or a diastereomeric stereochemical relationship between them, a...
What is a diastereomer?
Diastereomers are stereoisomers with two or more organic compounds that have at least two stereocenters with different configurations at some of the stereocenters but the same configuration at others.
How are diastereomers separated?
In terms of separation, diastereomers can be separated from one another by: Recrystallization: solids that are selectively crystallized in the presence of other compounds. Distillation: a method to separate liquid compounds from one another by taking advantage of their different boiling points.
What is the difference between stereocenter and stereoisomer?
Stereoisomers are compounds that have the same chemical formula , the same atom connectivity, but a different three-dimensional orientation or shape. A stereocenter is an atom (usually carbon) within a molecule that contains four different atoms or groups of atoms bonded to it.
How to separate diastereomers?
If the two compounds of interest are solids, a lot of times they can be separated by recrystallization, a technique in which solids can be selectively crystallized in the presence of other compounds. Another method that can be used to separate diastereomers is distillation. Distillation is a method to separate liquid compounds from one another by taking advantage of their different boiling points.
When considering whether a pair of molecules is related as being diastereomers of one another, it's?
When considering whether a pair of molecules is related as being diastereomers of one another, it's important to examine each stereocenter within both molecules. If all of the stereocenters within two compounds are exactly opposite of one another they are simply mirror images of one another, making them not diastereomers. If some of the stereocenters within one of the compounds are the opposite in configuration but others are the same , these compounds are diastereomers.
Is chlorine a diastereomer?
Since the compounds are different in the configurations of the chlorine atoms but the same with the bromine atoms, they are diastereomers. Notice also how they are not mirror images of one another. Sometimes diastereomers can include compounds that are ring structures.
Introduction of Diastereomers
Stereoisomers have the same chemical formula, structural formula, and same sequence of connectivity but differ in the arrangement of atoms in space. They occur when two or more stereoisomers of a compound have different configurations. We can categorize stereoisomers into three types. Enantiomers, Diastereomers and conformers.
Definition
All the optical isomers that do not mirror images are called diastereomers. They are non-identical stereoisomers. They are also called Diastereoisomers. They are neither enantiomers nor conformers.
Properties of diastereomers
They can be separated from one another through techniques like fractional crystallization, fractional distillation, chromatography, etc.
Conclusion
So, Diastereomers are not mirrored images, non-superimposable, and optically active compounds with different configurations (R and S) at two or more chiral centers but not all. They have the same structural, chemical, and molecular formula. The base on which we distinguish them is the arrangement of their molecules in space.
What is the formation of diastereomers?
By analogy, the formation of diastereomers is observed for additions to other trigonal systems, such as olefins, which have a chiral center elsewhere in the mole cule. In these cases, if optically active starting materials are used, then the diastereomers will be optically active. If racemic starting materials are employed, the diastereomeric mixture will be optically inactive. In either case it is common to find different amounts of the two diastereomers.
How to make diastereomers?
One of the most direct ways to produce diastereomers is by addition reactions across carbon – carbon double bonds. If the structure of the olefin substrate is such that two new chiral centers are produced by the addition of a particular reagent across the double bond, then diastereomers will result. For example, the addition of HBr to Z-3-chloro-2-phenyl-2-pentene produces 2-bromo-3-chloro-2-phenylpentane as a mixture of four diastereomers. Assuming only Markovnikov addition, the diastereomers are produced by the addition of a proton to C-3 followed by addition of bromide to the carbocation intermediate at C-2. Since the olefin precursor is planar, the proton can add from either face, and since the carbocation intermediate is also planar and freely rotating, the bromide can add to either face to give diastereomeric products. The possibilities are delineated schematically (but not mechanistically) below.
How to understand stereoselectivity?
To understand the stereoselectivity that might be observed, it is first necessary to delineate the stereochemical possibilities. The existing chiral center can be either R or S or both. Addition to the carbonyl group can potentially occur from either face since it is planar. Thus, if the existing chiral center is of the R config-uration, the products of addition will have either the R,R or R,S configurations and are diastereomers. If the starting material has only the R configuration, each diastereomer is optically active because only one enantiomer is produced. If the existing chiral center is of the S configuration, the products of addition can be either S,S or S,R diastereomers. Each diastereomer will be optically active, and they are enantiomers of the diastereomeric pair formed from the R configura-tion of the precursor. If the starting material is a racemic mixture, then all four stereoisomers will be produced —two sets of enantiomeric diastereomers. (That is, the R will give R,R and R,S and the S will give S,S and S,R.) The product mixture will be optically inactive.
What type of reaction produces diastereomeric mix-tures?
As stated previously, the addition of nucleophiles to chiral carbonyl com-pounds is a very common type of reaction which produces diastereomeric mix-tures. The diastereoselectivity varies with the reagents and conditions. Some examples are
What is the de% of diastereoselectivity?
The de% is given by de% = % major diastereomer − % minor diastereomer. For diastereospecific reactions in which a single diastereomer is produced, de = 100%, while for reactions in which there is no selectivity and diastereomers are produced in equal amounts, de = 0%.
How does stereospecificity in hydrogenation work?
Stereospecificity in hydrogenation is gained by a surface-mediated delivery of the hydrogen atoms to one face of the olefin. Stereospecificity in both hydrobora-tion/oxidation and osmium tetroxide/reduction results from a concerted addition to one face of the π system. This mode of addition guarantees that both new bonds are formed on the same face of the olefin. Although the reagents can add to either face of the olefin, this leads only to enantiomers of a single diastereomer. The concerted addition is the key feature which assures syn selectivity.
Does bromination have stereospecificity?
The stereospecific ity observed in olefin bromination is only possible if the inherent facial relationship of the olefinic bond is maintained throughout the addition process and only one bromine atom adds to each face. In bromination, the electrophilic addition leads to a bridged bromonium ion which not only maintains the initial olefin geometry but also forces the second bromine to add from the opposite direction (anti) .
Why are cis and trans isomers diastereomers?
Because the connectivity of atoms is the same and the arrangement is different, these are stereoisomers. Specifically, because they are not mirror images, we classify them as diastereomers. So, cis and trans isomers are diastereomers.
What is the difference between constitutional isomers and stereoisomers?
Notice the difference with constitutional isomers – in stereoisomers, the atoms are connected the same , however , some of them have a different arrangement. In the first pair, the Br is on position 2, but it is pointing towards you and away from on the second molecule.
What are enantiomers?
Enantiomers are two molecules that are nonsuperimposable mirror images: And this is what we had for our pairs of stereoisomers, they were nonsuperimposable mirror images – enantiomers. The lesson is that enantiomers are stereoisomers.
What is the simplest class of isomers?
Notice that the atoms are connected differently. And this is the simplest class of isomers which we call Constitutional (Structural) Isomers.
Do isomers have the same chemical formula?
Iso- means same, so, in order for any two molecules to be isomers, they must have the same chemical formula. But, of course, not any structures with the same chemical formula are isomers, as they may just be two different drawings of the same compound. Therefore, isomers are different compounds with the same chemical formula.
Is C a chiral molecule?
Unlike, these two, c represents two chiral molecules which are enantiomers.
Is a stereoisomer an enantiomer?
Now, not all the stereoisomers are enantiomers. Stereoisomers are also divided into two main groups.
What are the diastereomers?
I mean, seriously, the “common” definition of a diastereomers is the stereoisomers that are not enantiomers. The official definition though is the diastereomers are non-superimposable molecules that are not mirror images of each other. For instance, let’s look at the following two molecules:
What is diastereomer in biology?
The official definition though is the diastereomers are non-superimposable molecules that are not mirror images of each other. For instance, let’s look at the following two molecules: Molecules (3) and (4) are obviously not mirror images, so they cannot be enantiomers.
What are the two stereochemical relationships between two molecules?
Enantiomers and diastereomers are the only two stereochemical relationships that you can have between any two molecules. The stereoisomers are any two molecules that fulfill the following two requirements: Both molecules must have the same atom connectivity.
What is the difference between enantiomers and diastereomers?
Notice, that unlike enantiomers, diastereomers only have some of the stereocenters change from one molecule to the other. For instance, molecule (3) is ( 1S, 2R )-2-bromocyclopentanol, while its diastereomer is ( 1S, 2S )-2-bromocyclopentanol.
What are enantiomers?
Enantiomers are two molecules that are non-superimposable mirror images. Just like your hands, molecules may have a mirror image that won’t be superimposable with the original molecule. Look at these two molecules:
Do stereochemical relationships focus on chiral atoms?
Many students tend to have a sort of a tunnel vision when it comes to stereochemical relationships focusing only on molecules with chiral atoms. This is a faulty heuristic! So, make sure you always analyze the entire molecule and use the definition of the relationship, rather than only focusing on the chiral atoms.
Do you have to have chiral carbons to have enantiomers and diastereomers?
No, you do not! Note how the definition of enantiomers says that the molecules are non-superimposable mirror images, while diastereomers are non-superimposable non-mirror images? The definitions say nothing about the chiral centers or atoms. Thus, any pair of molecules that fits the definition, works! For instance, allenes are cumulated alkenes that are not planar:
What are stereoisomers?from chemistry.coach
Stereoisomers that are mirror images of each other and are non-superposable. 2 enantiomers have opposite configurations in all chirality centers. 2 enantiomers of a given compound have identical properties (melting point, solubilities, densities ...)
What are stereoisomers that are not mirror images of each other?from chemistry.coach
Diastereomers: Stereoisomers that are not mirror images of each other. At least one of their chirality center has an opposite configuration, but not all of them. 2 diastereomers have different properties. non-superposable mirror images. opposite configurations at all C*. ⇒ enantiomers.
What is the difference between a cis and a trans isomer?from chem.libretexts.org
In the cis isomer the methyl groups are on the same side; whereas they are on opposite sides in the trans isomer. Isomers that differ only in the spatial orientation of their component atoms are called stereoisomers. Stereoisomers always require that an additional nomenclature prefix be added to the IUPAC name in order to indicate their spatial orientation, for example, cis (Latin, meaning on this side) and trans (Latin, meaning across) in the 2-butene case.
What is an enantiomer?from khanacademy.org
Enantiomers are stereoisomers that are non-superimposable mirror images. Enantiomers differ at the configuration of every stereocenter. They can be understood in terms of handedness, like gloves for the right or left hands.
How many chiral centres does 1,3-dimethylcyclopentane have?from khanacademy.org
In fact, 1,3-dimethylcyclopentane has only 2 chiral centres and 3 distinct stereoisomers. Try drawing the molecule out with the two methyl groups trans to one another (both up), you will then notice that there is a centre of symmetry - This "trans" form is a mess compound. If you then draw the two cis versions by drawing one methyl up ...
Why are enantiomers different from each other?from khanacademy.org
In this tutorial, we will explore enantiomers, compounds that have the same composition and bonding but are fundamentally different because they are mirror images of each other (kind of like Tomax and Xamot--the Crimson Guard Commanders from GI Joe).
What is a meso compound?from chemistry.coach
Meso compounds = compounds that are achiral, yet contain chirality centers. #N#They do not have optical activity: [α] D = 0°.#N#A meso compound has a plane of symmetry and is superimposable on the compound with opposite configurations at all chirality centers.
What are Diastereomers?from byjus.com
Diastereomers are defined as compounds which have the same molecular formula and sequence of bonded elements but which are nonsuperimposable, non-mirror images.
How to separate diastereomers?from byjus.com
On account of differences in their physical properties, diastereomers can be separated from one another through techniques like fractional crystallization, fractional distillation, chromatography etc. The difference from enantiomers which can’t be separated by these techniques.
How do diastereomers differ from enantiomers?from en.wikipedia.org
Diastereomers differ from enantiomers in that the latter are pairs of stereoisomers that differ in all stereocenters and are therefore mirror images of one another. Enantiomers of a compound with more than one stereocenter are also diastereomers of the other stereoisomers of that compound that are not their mirror image (that is, ...
What happens if there are more than one chiral center?from byjus.com
If there is more than one chiral center in a molecule, you have the risk of making stereoisomers not mirroring each other’s images. These stereoisomers are called diastereomers, which are not mirror images. Typically, diastereomers can only be detected when the molecule has two or more chiral centres.
What are the two types of stereoisomers?from byjus.com
Enantiomers and diastereomers are two types of stereoisomers. Enantiomers include mirror images and non-superimposable chiral centers. Diastereomers contain non-superimposable chiral centers, but are not mirror images. Depending on the number of stereocenters, there could be far more than 2.
What is threo in biology?from byjus.com
It is called threo if similar groups are on the opposite sides of the Fischers projection. For example, hydroxylation of trans-crotonic acid gives two enantiomers of the threo-2,3-dihydroxybutanoic acid whereas the same reaction with cis-crotonic acid gives the erythro enantiomers.
How many chiral centres does a diastereomer have?from byjus.com
Remember that pairs of diastereomers exist, and each has two chiral centres. The chirality of one of them would be (for example) “R, S” in the original classic diastereomer, and the other would be “R, R.”.
What are constitutional isomers and stereoisomers?
The terms constitutional isomers and stereoisomers make up two broad categories of isomers (molecules with the same chemical formula). Constitutional iso mers typically have different connectivities and stereoisomers have the same connectivities but differ in spatial arrangements.
What is an enantiomer?
enantiomers are stereoisomers that are non-superimposeable mirror images of one another. Your hands are (roughly) enantiomers. both are optically active is true of enantiomers.
Is cis- and trans-dichloroethene an enantiomer?
Your hands are (roughly) en antiomers. both are optically active is true of enantiomers. diastereomers also known as geometric isomers are also stereoisomers that are not mirror images of one another. cis- and trans-dichloroethene are diastereomers.