What are 5 examples of covalent bonds?
What are 5 examples of covalent bonds? Hydrogen (H 2) Hydrogen (H) is the simplest of all elements. … Oxygen (O 2) The valency of oxygen (O) is two, which means that it requires two electrons to complete its outermost (valence) shell. … Nitrogen (N 2) … Water (H 2 O) … Carbon Dioxide (CO 2) … Methane (CH 4) … Ammonia (NH 3) …
How do you describe a covalent bond?
Types of Covalent Bonds
- During the formation of a covalent bond between two atoms, each atom contributes 1, 2 or 3 electrons to each other for sharing.
- By doing so, the two atoms share 1, 2 or 3 pairs of electrons so as to achieve stable noble gas electron arrangements.
- When two atoms share one pair of electrons, a single covalent bond is formed.
- When two atoms share two pairs of electrons, a double covalent bond is formed.
Does a covalent bond have a charge?
This unequal distribution of electrons is known as a polar covalent bond, characterized by a partial positive charge on one atom and a partial negative charge on the other. The atom that attracts the electrons more strongly acquires the partial negative charge and vice versa.
How to do covalent bonding?
Covalent bonding. The atoms form a covalent bond by sharing their valence electrons to get a stable octet of electrons.(filled valence shell of 8 electrons) There are two electrons per bond, each atom donates one electron to the bond. Electron-Dot Diagrams of the atoms are combined to show the covalent bonds. Covalently bonded atoms form MOLECULES

What is a covalent bond Class 8?
A covalent bond is formed by equal sharing of electrons from both the participating atoms. The pair of electrons participating in this type of bonding is called shared pair or bonding pair. The covalent bonds are also termed as molecular bonds.
What is covalent bonding GCSE AQA?
A covalent bond is a shared pair of electrons. Covalent bonding results in the formation of molecules or giant structures. Substances with small molecules have low melting and boiling points and do not conduct electricity. Giant covalent substances have very high melting points.
How is the covalent bond formed class 8th?
As pairs of electrons are exchanged between atoms, covalent bonding happens. In order to achieve further stability, which is gained by forming a complete electron shell, atoms can covalently bond with other atoms.
What is covalent bond in class 11th?
A covalent bond, also called a molecular bond, is a chemical bondthat involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs orbonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.
How do you define a covalent bond?
A covalent bond consists of the mutual sharing of one or more pairs of electrons between two atoms. These electrons are simultaneously attracted by the two atomic nuclei. A covalent bond forms when the difference between the electronegativities of two atoms is too small for an electron transfer to occur to form ions.
What is covalent bond one word answer?
A covalent bond is a chemical bond that involves the sharing of electrons to form electron pairs between atoms.
What is covalent bond Ncert?
Covalent bond between two Cl atoms. Thus, when two atoms share one electron pair they are said to be joined by a single covalent bond. In many compounds we have multiple bonds between atoms. The formation of multiple bonds envisages sharing of more than one electron pair between two atoms.
What is covalent bond BYJU's?
A covalent bond: The chemical bond formed due to mutual sharing of electrons between the given pairs of atoms of nonmetallic elements is called a covalent bond. In covalent bond, atoms share electrons to complete their duplets or octets.
What is covalent give its examples?
Covalent compounds are compounds that are formed by the sharing of electrons between two or more atoms. Example: Hydrogen (H2) is a covalent compound, where each hydrogen atom shares one electron to form a stable compound.
What is a ionic bond Class 11?
An Ionic bond is the bond formed by the complete transfer of valence electron to attain stability. This type of bonding leads to the formation of two oppositely charged ions – positive ions known as cations and negative ions known as anions.
What is ionic bonding AQA GCSE?
When metals react with non-metals, electrons are transferred from the metal atoms to the non-metal atoms, forming ions. The resulting compound is called an ionic compound. Reactions between metals and non-metals include: sodium + chlorine → sodium chloride.
What is covalent bonding GCSE Edexcel?
A covalent bond is formed when a pair of electrons is shared between two atoms , usually non-metals .
What is bonding in chemistry GCSE?
Compounds are substances in which atoms of two, or more, elements are not just mixed together but chemically combined. This is known as Bonding. Chemical reactions between elements involve either the giving and taking, or sharing, of electrons in the highest occupied energy levels of atoms.
What is a ionic bond GCSE?
Ionic bonding - when metals and nonmetals give/take electrons from each other. Covalent bonding - when nonmetals share electrons with other non-metals. Metallic bonding - when metals form a lattice of positive ions surrounded by a sea of electrons.
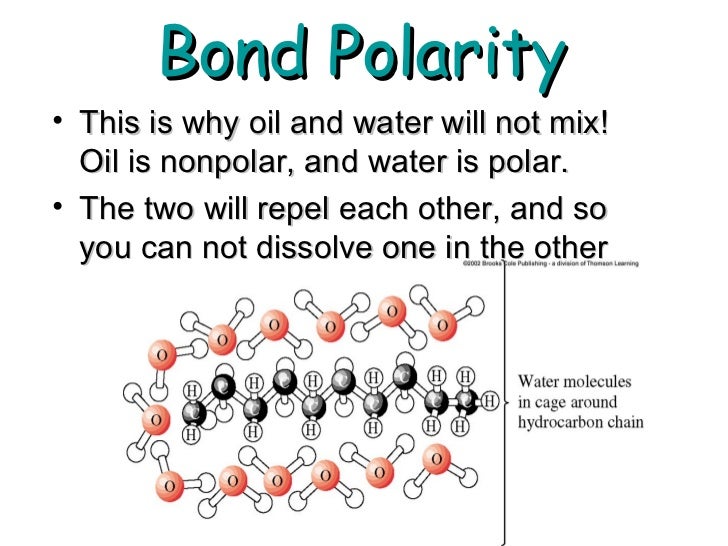
What is polar covalent bond?
A polar covalent bond, known also as a polar bond, is a covalent bond between atoms in which the electrons are shared unequally.
How are valence electrons arranged?
based off the VSPER theory, the pairs of valence electrons are arranged as far apart from each other as possible, meaning that the unshared pairs are repulsed as far as possible
How many pm is each Br atom in a covalent bond?
Each Br atom in a covalent bond is 114 pm. Use that information along with the length of the C–Br bond in CBr4 to determine the C atom's radius:
How do ionic and covalent bonds differ?
An ionic bond and a covalent bond differ in the location of the electrons. In ionic bonds, there is no sharing of electrons. The cation is deficient in electrons and the anion has extra electrons. The opposite charges of these ions cause them to be attracted to each other and that is what we call ionic bonding. In contrast, when both atoms want to gain electrons, they achieve this by sharing electrons. The atoms must stay close together to share electrons; hence, a different type of bond – a covalent bond – is formed.
How many valence electrons does Cl3+ have?
(b) The Cl3+ ion has 20 valence electrons, just as in (a). One of the Cl atoms is the central atom, while the other two Cl atoms are single bonded to the central Cl atom: .. ....+Cl Cl.. Cl
How many electrons does the central atom have?
The central atom, C, has only six electrons in shared pairs, so it needs two more, but there are no more electrons available for lone pairs. Therefore, we must move a lone pair of electrons from one of the O atoms to make a new shared pair, forming a double bond to the C atom. The atomic arrangement given in the problem tells us to put the multiple bond on the top oxygen: ..
What is the bonding pattern of Structure 7?
In Structure 7, we see a positive formal charges occurring on adjacent atoms, and one of these positive formal charges is found on a highly electronegative O atom. These two things makes this bonding pattern very unlikely. Therefore we won't bother checking the formal charges of any of the rest of the possible structures that have a triple bond to an O atom.
How to write a structural formula for a branched chain?
Develop a plan: Be systematic. Start with a long chain that has only one methyl branch. Move the methyl around (but don't put it on the end carbon and don't put it on a carbon past the first half of the chain). Then make two methyl branches, and move them around similarly. Continue this process until the main chain is too short for methyl branches. Then make an ethyl branch and move it around (but don't put it on the end carbon or the carbon next to the end carbon and don't put it on a carbon past the first half of the chain). Follow a similar pattern with ethyls as with methyls.
