
Which particles in atoms have a positive charge?
Summary
- Electrons are a type of subatomic particle with a negative charge.
- Protons are a type of subatomic particle with a positive charge. ...
- Neutrons are a type of subatomic particle with no charge (they are neutral). ...
What does it mean if an atom is positively charged?
When an atom or group of atoms has more protons than electrons, it is positively charged. An atom or group of atoms that has the same number of protons and electrons is neutrally charged.
Which particle of the atom has a negative charge?
The electron is the lightest stable subatomic particle known. It carries a negative charge which is considered the basic charge of electricity. An electron is nearly massless.
What are particles around the atom have negative charge?
What are the properties of subatomic particles?
- Proton (p +) is positively charged particle of the atomic nucleus. …
- Electron (e –) is negatively charged particle that can occupy a volume of space (orbital) around an atomic nucleus. …
- Atoms have an equal number of protons and electrons; therefore, they have a no net charge.
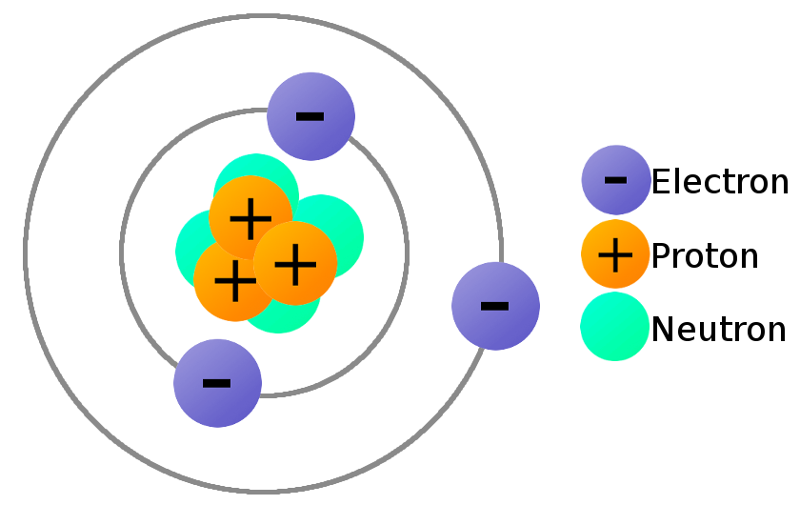
What are positively charged atoms?
The atom that has lost an electron becomes a positively charged ion (called a cation), while the atom that picks up the extra electron becomes a negatively charged ion (called an anion).
What is known as positively charged particle?
Proton is the positively charged particle present in atoms of all the elements.
What are the charged particles of an atom called?
An atom consists of a positively charged nucleus, surrounded by one or more negatively charged particles called electrons.
Why is an atom positively charged?
Heavier atoms tend to have more neutrons than protons, but the number of electrons in an atom is always equal to the number of protons. So an atom as a whole is electrically neutral. When one or more electrons is stripped away from an atom, it becomes positively charged.
Why is proton positively charged?
The charge is believed to be from the charge of the quarks that make up the nucleons (protons and neutrons). A proton is made of two Up quarks, with 2/3 positive charge each and one Down Quark with a negative 1/3 charge (2/3 + 2/3 + -1/3 = 1).
Which is found in the nucleus of an atom?
Atomic nuclei consist of electrically positive protons and electrically neutral neutrons. These are held together by the strongest known fundamental force, called the strong force. The nucleus makes up much less than . 01% of the volume of the atom, but typically contains more than 99.9% of the mass of the atom.
Is electron a charged particle?
electron, lightest stable subatomic particle known. It carries a negative charge of 1.602176634 × 10−19 coulomb, which is considered the basic unit of electric charge.
What are negatively charged particles called?
ElectronsElectrons are negatively charged subatomic particles found in the outermost regions of atoms.
What are uncharged particles?
Neutron: An uncharged particle found in the nucleus of an atom. A neutron, like a proton, contributes one atomic mass unit to the total atomic weight of an atom.
What are the particles in an atom that do not have any charge called?
neutron, neutral subatomic particle that is a constituent of every atomic nucleus except ordinary hydrogen. It has no electric charge and a rest mass equal to 1.67493 × 10−27 kg—marginally greater than that of the proton but nearly 1,839 times greater than that of the electron.