What are the different types of redox reactions?
Types of Redox Reactions
- Decomposition Reaction. This kind of reaction involves the breakdown of a compound into different compounds. ...
- Combination Reaction. These reactions are the opposite of decomposition reaction and hence involve the combination of two compounds to form a single compound in the form of A + B ...
- Displacement Reaction. ...
- Disproportionation Reactions. ...
What type of reaction is always a redox reaction?
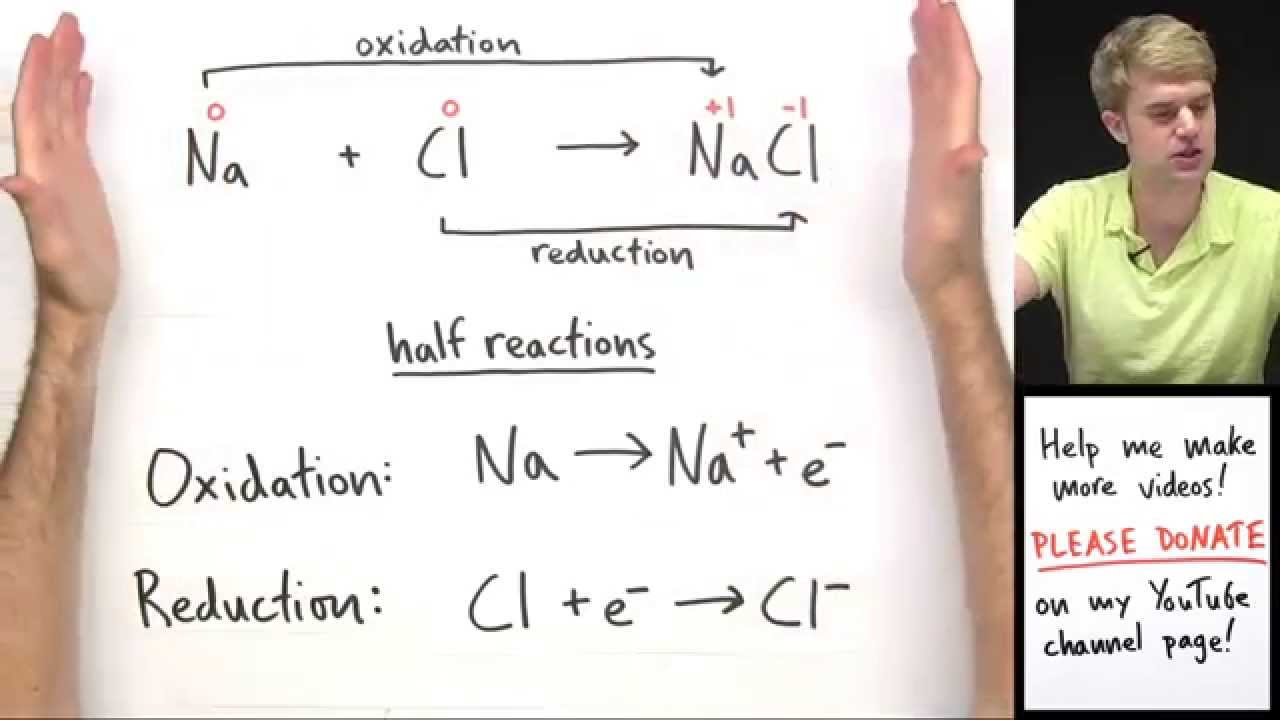
The term redox refers to the reduction-oxidation process. All redox reactions can be divided into two types of processes: reduction and oxidation. The redox reaction, also known as the Oxidation-Reduction reaction, always involves simultaneous oxidation and reduction events.
What are some of the applications of a redox reaction?
- A voltaic cell produces electricity as a redox reaction occurs.
- The voltage of a voltaic cell can be determined by the reduction potentials of the half reactions.
- Voltaic cells are fashioned into batteries, which are a convenient source of electricity.
What is meant by a redox reaction?
Redox reactions are reactions in which one species is reduced and another is oxidized. Therefore the oxidation state of the species involved must change. These reactions are important for a number of applications, including energy storage devices (batteries), photographic processing, and energy production and utilization in living systems including humans.

What is redox reaction explain with example?
The oxidation and reduction occurring together are called a redox reaction. CuO+H2→Cu+H2O. Example: In this reaction, copper oxide is being reduced to copper whereas hydrogen is being oxidised to water.
What are the 3 types of redox reaction?
Redox reactions can be primarily classified into five different types:Combination Reactions.Decomposition Reactions.Displacement Reactions.Disproportionate Reactions.
What is a real life example of a redox reaction?
Combustion forms the classic example of redox reactions in real-life. However, whenever we talk about combustion, we usually view it as a physical change than a chemical one. The burning of organic material and combustion of hydrocarbons in fossil fuels form yet another important example of redox reactions.
What is the most common redox reaction?
Most common oxidation-reduction (redox) reactions are combination, decomposition, displacement, and combustion reactions that are listed below.
Which one is a redox reaction?
oxidation-reduction reaction, also called redox reaction, any chemical reaction in which the oxidation number of a participating chemical species changes.
Is breathing a redox reaction?
Complete Step By Step Answer: Cellular respiration is a redox reaction, which is an oxidation-reduction reaction. Respiration is a collection of metabolic reactions in which electrons are lost and gained. As a result, it's referred to as the oxidation-reduction or redox reaction.
How do you identify redox reactions?
A redox equation can be balanced using the following stepwise procedure: (1) Divide the equation into two half-reactions. (2) Balance each half-reaction for mass and charge. (3) Equalize the number of electrons transferred in each half-reaction. (4) Add the half-reactions together.
What are 2 examples of oxidation?
For example, iron metal is oxidized and forms an iron oxide which is known as rust. It reduces oxygen. Production of magnesium oxide by the reaction of magnesium and oxygen is also another example of oxidation where an element is reacting with oxygen.
1. Is every chemical reaction a redox reaction?
No, every chemical reaction is not a redox reaction. Redox reaction is a reaction that includes both oxidation and reduction. Reactions like Acid-B...
2. How to determine if Redox reactions have occurred?
A Redox reaction involves the oxidation and reduction of the elements. To find chemical reactions that are redox, compare the oxidation numbers fro...
3. What is the importance of Redox reactions in human lives?
Redox reactions give fundamental energy that drives or supports human life. Reactions that occur during photosynthesis and cellular respiration are...
4. Is displacement reaction a redox reaction?
When a more reactive metal displaces a less reactive metal from its salt, it is called a displacement reaction. In these reactions, you determine p...
What happens to the reducing agent in a redox reaction?
In redox reactions reducing agent always convert into its conjugating oxidizing agent in an oxidation – reducing reaction. Thus, the products of this reaction will include a new oxidizing agent and a new reducing agent. It is represented below in the reaction –. (image will be uploaded soon)
What is a reducing agent?
Reducing agent can be defined as those groups which gain oxygen atom from the substrate (or oxidizing agent) although it is not true in every case as in many redox reactions, oxidation – reduction takes place in absence of oxygen atoms. Examples of these reducing agents include formic acid, oxalic acid, sulfites etc.
What are some examples of oxidizing agents?
Oxygen, hydrogen peroxide and halogens are examples of some common oxidizing agents. The oxidizing agent can be defined as those groups which transfer oxygen atom to the substrate although its not true in every case as in many redox reactions, oxidation – reduction takes place in absence of oxygen atoms as well.
What is a redox reaction?
What are Redox Reactions? The term redox is made up of two words reduction and oxidation. A type of chemical reaction that involves a transfer of electrons between two species is called redo x reaction. In these types of reactions oxidation and reduction both take place together.
What is disproportionation reaction?
Disproportionation Reactions – These are special type of redox reactions. In these reactions an element in one oxidation state is simultaneously oxidized and reduced. In these reactions one of the reacting substances always contains an element that can exist in at least three oxidation states.
What method is used to balance redox reactions?
Balancing of Redox Reactions - For balancing the redox reactions oxidation number method and half reaction method are used.
How does glucose turn into carbon dioxide?
Glucose gets oxidized into carbon dioxide by losing hydrogens while oxygen gets reduced into water by gaining hydrogens. reaction is given below –. (image will be uploaded soon) Combustion – Combustion is an exothermic redox chemical reaction which occurs at high temperature and in presence of an oxidant.
What is a redox reaction?
What is Redox Reaction. Many chemical reactions involve transfer of electrons from one chemical substance to another. These electron transfer reactions are termed as oxidation-reduction or Redox reaction, or those reactions which involve oxidation and reduction both simultaneously are known as oxidation and reduction/ Redox reaction.
What are reducing agents?
Reducing Agents or Reductant. The substances which donate electrons in a chemical reaction are reducing agents i.e electron donors are reducing agents. Reducing agents are Lewis base. Substances which can reduce others and oxidise themselves. Substance which shows decrement in oxidation number.
What are the different types of redox reactions?
There are mainly three types of redox reactions, they are: 1.Intermolecular Redox Reaction. When oxidation and reduction take place separately in the different compounds called intermolecular redox reactions. SnCl2+ 2FeCl3-------SnCl4+2FeCl2.
What is the term for the transfer of electrons from one chemical substance to another?
Many chemical reactions involve transfer of electrons from one chemical substance to another. These electron transfer reactions are termed as oxidation-reduction or Redox reaction, or those reactions which involve oxidation and reduction both simultaneously are known as oxidation and reduction/ Redox reaction.
What is reduction in chemistry?
Reduction is a process which involves: Removal of oxygen. Addition of hydrogen. Removal of electronegative elements. Addition of electropositive elements. Decrement in oxidation state of electropositive elements.
Which substance accepts electrons in a chemical reaction?
The Substance which accept electrons in a chemical reaction i.e electron acceptors are oxidising agents: Oxidising agents are lewis acid. Substance which can oxidise others and reduce themselves. Substance which shows decrement in oxidation number.
What are the four concepts of oxidation and reduction?
Oxidation is a process which involves: Addition of oxygen. Removal of hydrogen. Addition of electronegative elements. Removal of electropositive elements .
What is the oxidation number of chlorine?
Let us understand this through an example. The oxidation number of chlorine in hydrochloric acid (HCl) is -1, in Chloric acid (HClO 3) is +5, and in perchloric acid (HClO 4) it is +7.
What are some examples of redox reactions?
Redox reactions, in fact, play a crucial role in biochemical reactions, industrial processes, and other chemical works. Transfer of cells and glucose oxidation in the body are also classic examples of these type of reactions. Reactions in chemical factories, electrochemical reactions, obtaining metals from their ores, ...
What is the reaction of an acid and a base?
Reaction of an acid and a base: 3 CuS + 8 HNO 3 → 3 CuSO 4 + 8 NO (g) + 4 H 2 O
How to find oxidation number?
How do we calculate oxidation number? Well, for calculating oxidation number, one has to consider various oxidation states of all atoms in a molecule and then equate the sum of all of them to the net charge on the molecule. There are various rules for finding oxidation number of an atom in a molecule however, here we will just see how to find oxidation number of Cl in HClO 3.
What is redox in chemistry?
The word ‘redox’ is the acronym for reduction-oxidation that occurs in a certain chemical reaction. Put more simply, these reactions are those sets of chemical equations in which the oxidation number of the atoms involved in the chemical reaction changes when the reaction occurs. This definition introduces us to another fundamental concept in chemistry that is of crucial importance, the oxidation number. Let us define it.
What are the changes that occur during oxidation?
So on a general note, oxidation generally involves any of the following changes: Loss of electrons. Loss of hydrogen atoms. Gain of oxygen. Increase in oxidation state. Similarly, reduction is said to occur when any of the following changes occur: Gain of electrons. Gain of hydrogen atoms. Loss of oxygen atoms.
How does hydrogen oxidize?
In the first reaction, hydrogen oxidizes by increasing its oxidation number from 0 to +1 while in the second reaction, fluorine is reduced by decreasing its oxidation number from 0 to -1. Eventually, net charge on the molecules formed is zero as number of electrons gained during oxidation are consumed during reduction process.
