What are the components of nitrogen?
Feb 24, 2020 · You can view more details on each measurement unit: molecular weight of Nitrogen or grams The molecular formula for Nitrogen is N. The SI base unit for amount of substance is the mole. 1 mole is equal to 1 moles Nitrogen, or 14.0067 grams.
Is nitrogen a molecule or an atom?
nitrogen unit. 1. n. [Well Completions] A high- pressure pump or compressor unit capable of delivering high-purity nitrogen gas for use in oil or gas wells. Two basic types of unit are commonly available: nitrogen converter unit that pumps liquid nitrogen at high pressure through a heat exchanger or converter to deliver high-pressure gas at ambient temperature.
What converts nitrogen in the nitrogen cycle?
Jul 17, 2017 · Pounds are the most common unit, but you could use grams instead if you prefer. A unit of nitrogen fertilizer is just one unit of whichever unit you choose to describe the nitrogen content. Calculating cost per unit of nitrogen gives you a …
What are the common uses of nitrogen?
Mar 21, 2016 · UAN32 weighs 11.06 pounds per gallon, for a calculation of 11.06 pounds x 0.32 = 3.5 pounds. So if you want to apply 140 pounds N, and use a split application, you would apply 20 gallons UAN32 in...
See more
Nov 21, 2020 · Nitrogen – Atomic Mass – Atomic Weight – N 2020-11-21 by Nick Connor Atomic Mass of Nitrogen Atomic mass of Nitrogen is 14.0067 u.

How do you calculate units of nitrogen?
To calculate the pounds of nitrogen in a bag of fertilizer, multiply the weight of the bag by the percent nitrogen (this is the first number in the N-P-K designation on the front of the bag). This will tell you the pounds of nitrogen in the bag.
How much does a unit of nitrogen cost?
Nitrogen cost in 1 ton of 28% UAN = $225 ÷ 560 lbs N = 40 cents per lb N. Nitrogen cost in 1 ton of 10-34-0 = $70 ÷ 200 lbs N = 35 cents per lb N.
How many units of nitrogen are in a gallon?
You get 3.54 Units of Actual N out of that one gallon that is 11.06 pounds.Jul 7, 2011
What are fertilizer units?
A unit is the same as kilograms of that nutrient (eg 1 unit P is 1 kg P). But this is not the same as a kilogram of a fertiliser. N:P:K:S. Fertilisers contain different amounts of nutrients, affecting the amount of the fertiliser you need.
What is the cheapest nitrogen fertilizer?
Urea: A Low Cost Nitrogen Fertilizer with Special Management Requirements. Urea (46-0-0) usually has the lowest cost per pound of nitrogen compared to other single-element nitrogen fertilizers.
How do you calculate nitrogen per acre?
Calculate the pounds of fertilizer per acre you must use to apply 50 pounds of nitrogen per acre (N/A). To do this, divide the amount of the nutrient wanted by the proportion of that nutrient in the fertilizer used.Jun 14, 2016
Is a unit of nitrogen the same as a pound?
If the N-P-K rating is 10-6-4, for example, and the weight of the bag of fertilizer is 25 pounds, the fertilizer bag contains 2.5 pounds of nitrogen. The one unit of nitrogen in this case would be 1 pound.Jul 17, 2017
How many units of nitrogen are in a lb of urea?
Urea contains 920 actual pounds of nitrogen per ton, and ammonium nitrate contains 680 actual pounds of nitrogen by ton (2,000 x 46% = 920 and 2,000 x 34% = 680). How much actual nitrogen is in a fertilizer with an analysis of 27-0-0?
How much is nitrogen per gallon?
1 gallon of UN-32 weighs 11.02 pounds. 32% of the weight is nitrogen – hence the “32.” This roughly equals 3.4 pounds of nitrogen per gallon, or 55 ounces.Mar 26, 2010
What is 28% liquid nitrogen?
UAN is a liquid fertilizer containing three forms of nitrogen: urea, ammonium-N and nitrate-N.
How much nitrogen is in liquid fertilizer?
There are 4 quarts in a gallon, so 9 pounds divided by 4 is 2.25 pounds of fertilizer liquid in 1 quart. Your product is 16 percent nitrogen by weight, so 16/100 = 0.16, and 0.16 x 2.25 is 0.36. This means that 1 quart of fertilizer supplies 0.36 pounds of nitrogen.
What is N-P-K stand for?
In short, fertilizers are labeled N, P or K to indicate their nutrient content in terms of nitrogen (N), phosphorus (P), and potassium (K). All three are important for plant growth.
Where did the word nitrogen come from?
The English word nitrogen (1794) entered the language from the French nitrogène, coined in 1790 by French chemist Jean-Antoine Chaptal (1756–1832), from the French nitre ( potassium nitrate, also called saltpeter) and the French suffix -gène, "producing", from the Greek -γενής (-genes, "begotten").
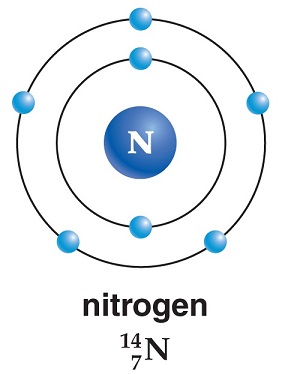
How many electrons does a nitrogen atom have?
From left to right: 1s, 2s (cutaway to show internal structure), 2p x, 2p y, 2p z. A nitrogen atom has seven electrons. In the ground state, they are arranged in the electron configuration 1s 2. 2s 2. 2p 1.
What are some examples of dinitrogen complexes?
The first example of a dinitrogen complex to be discovered was
How is nitrogen gas produced?
Nitrogen gas is an industrial gas produced by the fractional distillation of liquid air, or by mechanical means using gaseous air (pressurised reverse osmosis membrane or pressure swing adsorption ). Nitrogen gas generators using membranes or pressure swing adsorption (PSA) are typically more cost and energy efficient than bulk delivered nitrogen. Commercial nitrogen is often a byproduct of air-processing for industrial concentration of oxygen for steelmaking and other purposes. When supplied compressed in cylinders it is often called OFN (oxygen-free nitrogen). Commercial-grade nitrogen already contains at most 20 ppm oxygen, and specially purified grades containing at most 2 ppm oxygen and 10 ppm argon are also available.
What is the name of the mixture of nitric and hydrochloric acids?
The mixture of nitric and hydrochloric acids was known as aqua regia (royal water), celebrated for its ability to dissolve gold, the king of metals. The discovery of nitrogen is attributed to the Scottish physician Daniel Rutherford in 1772, who called it noxious air.
Why do we use nitrogen in aircraft fuel?
In some aircraft fuel systems to reduce fire hazard (see inerting system ). To inflate race car and aircraft tires, reducing the problems of inconsistent expansion and contraction caused by moisture and oxygen in natural air. Nitrogen is commonly used during sample preparation in chemical analysis.
What is the nitrogen cycle?
The nitrogen cycle describes movement of the element from the air, into the biosphere and organic compounds, then back into the atmosphere.
Types
Most fertilizers contain the three elements nitrogen, phosphorus and potassium. The ratio between these elements in the product is called the N-P-K rating and is usually expressed as three numbers separated by dashes — 10-6-4, for example. The first number is the nitrogen content.
Function
To find the nitrogen content of a fertilizer, multiply the number in the N position of the N-P-K rating by the total weight of the fertilizer. If the N-P-K rating is 10-6-4, for example, and the weight of the bag of fertilizer is 25 pounds, the fertilizer bag contains 2.5 pounds of nitrogen. The one unit of nitrogen in this case would be 1 pound.
Misconceptions
Which unit you use to measure nitrogen content isn't important as long as you are consistent. Pounds are the most common unit, but you could use grams instead if you prefer. A unit of nitrogen fertilizer is just one unit of whichever unit you choose to describe the nitrogen content.
How much does UAN32 weigh?
UAN32 weighs 11.06 pounds per gallon, for a calculation of 11.06 pounds x 0.32 = 3.5 pounds. So if you want to apply 140 pounds N, and use a split application, you would apply 20 gallons UAN32 in the spring (70 pounds divided by 3.5 pounds per gallon) and 24 gallons UAN28 sidedress (70 pounds divided by 2.9 pounds per gallon).
How much ammonia to apply per acre?
So if you want to apply 140 pounds N per acre, you apply 171 pounds of ammonia (140 pounds divided by 0.82 = 171 pounds). Liquids get a bit more complicated because you are dealing with volume and weight.
Is ammonia a gas or a liquid?
Anhydrous ammonia is a gas but a liquid under pressure. The liquid converts back to a gas once it leaves the manifold. Ammonia is quite easy to calculate because you are dealing with weight not liquid so the calculation works the same as for urea. Ammonia has an analysis of 82-0-0.
How to calculate pounds of nitrogen in fertilizer?
Additionally, how do you calculate pounds of nitrogen in fertilizer? To calculate the pounds of nitrogen in a bag of fertilizer, multiply the weight of the bag by the percent nitrogen (this is the first number in the N-P-K designation on the front of the bag). This will tell you the pounds of nitrogen in the bag.
What is the impurity in urea fertilizer?
A common impurity in urea fertilizers is biuret (C2H5N3O2), which can be broken-down in soil but does so over a long period of time, and is phytotoxic during the process. Similar Asks.
Overview
Chemistry and compounds
Atomic nitrogen, also known as active nitrogen, is highly reactive, being a triradical with three unpaired electrons. Free nitrogen atoms easily react with most elements to form nitrides, and even when two free nitrogen atoms collide to produce an excited N2 molecule, they may release so much energy on collision with even such stable molecules as carbon dioxide and waterto cause …
History
Nitrogen compounds have a very long history, ammonium chloride having been known to Herodotus. They were well known by the Middle Ages. Alchemists knew nitric acid as aqua fortis (strong water), as well as other nitrogen compounds such as ammonium salts and nitrate salts. The mixture of nitric and hydrochloric acids was known as aqua regia (royal water), celebrated for its ability to dissolve
Properties
A nitrogen atom has seven electrons. In the ground state, they are arranged in the electron configuration 1s 2s 2p x2p y2p z. It therefore has five valence electrons in the 2s and 2p orbitals, three of which (the p-electrons) are unpaired. It has one of the highest electronegativities among the elements (3.04 on the Pauling scale), exceeded only by chlorine (3.16), oxygen (3.44), and fluorine(3.98…
Occurrence
Nitrogen is the most common pure element in the earth, making up 78.1% of the volume of the atmosphere. Despite this, it is not very abundant in Earth's crust, making up only 19 parts per million of this, on par with niobium, gallium, and lithium. The only important nitrogen minerals are nitre (potassium nitrate, saltpetre) and soda nitre (sodium nitrate, Chilean saltpetre). However, these ha…
Production
Nitrogen gas is an industrial gas produced by the fractional distillation of liquid air, or by mechanical means using gaseous air (pressurised reverse osmosis membrane or pressure swing adsorption). Nitrogen gas generators using membranes or pressure swing adsorption (PSA) are typically more cost and energy efficient than bulk delivered nitrogen. Commercial nitrogen is often a byproduct of air-processing for industrial concentration of oxygenfor steelmaking and other purposes. Whe…
Applications
The applications of nitrogen compounds are naturally extremely widely varied due to the huge size of this class: hence, only applications of pure nitrogen itself will be considered here. Two-thirds (2/3) of nitrogen produced by industry is sold as the gas and the remaining one-third (1/3) as the liquid.
The gas is mostly used as an inert atmosphere whenever the oxygen in the air …
Safety
Although nitrogen is non-toxic, when released into an enclosed space it can displace oxygen, and therefore presents an asphyxiation hazard. This may happen with few warning symptoms, since the human carotid body is a relatively poor and slow low-oxygen (hypoxia) sensing system. An example occurred shortly before the launch of the first Space Shuttle mission on March 19, 1981, when two technicians died from asphyxiation after they walked into a space located in the Spac…