
What do you need to know about ammonium nitrate?
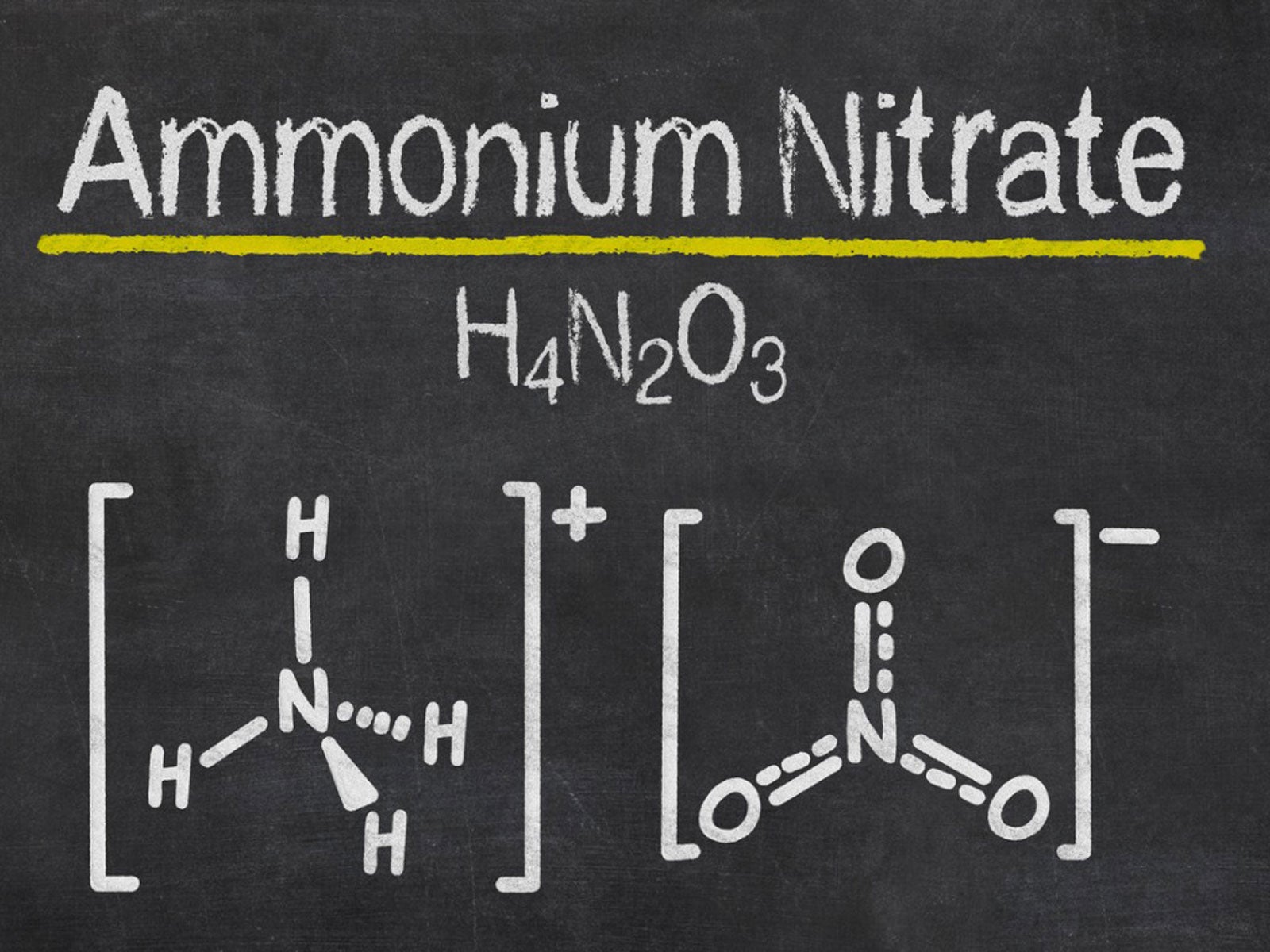
The chemical symbol of Ammonium nitrate is NH 4 NO 3 its molar mass is 80.043 g/mol, it has ionic bonding and contains two ions: a cation, the ammonium ion (NH 4+) and an anion, the nitrate ion (NO 3–) (Figure 2). The boiling point of ammonium nitrate is 210 and its melting point is 169.6 and it has a density of 1.72 g/cm 3 (Pubchem, 2018) .
Is ammonium nitrate an element compound or mixture?
Ammonium Nitrate. Pure Substance/Compound. Spaghetti and Meatballs. ... State a difference between a compound and an element. Compounds are different atoms combined, but element are same atoms combined. ... A homogeneous mixture of metals can also be referred to by this very specific name.
Does ammonium nitrate react with water?
It feels cold when ammonium nitrate is dissolved in water which indicates an endothermic reaction. In an endothermic reaction, the ammonium nitrate dissolves in water, a chemical reaction that absorbs heat rather than releases it. Ammonium nitrate consists of tightly packed, ionic bonds.
What does ammonium nitrate smell like?
Ammonium nitrate is a crystal salt consisting of ammonia and nitric acid. It is odorless and is either colorless or white. They are characterized by a yellow smoke and distinctive smell. Why do farmers use ammonium nitrate on their fields?
See more

Is ammonium nitrate used for explosion?
Ammonium Nitrate is not an explosive by itself. However, it is one of the ingredients used for manufacture of explosives. It is classified as an oxidizer (5.1) as per UN classification for Dangerous Goods. Other ingredients like fuel, etc., have to be added to make it an explosive.
Is ammonium nitrate used in weapons?
Is it used in bombs? With such a powerful blast, ammonium nitrate has been used by armies around the world as an explosive. It has also been used in several terrorist acts, including the Oklahoma City bombing in 1995.
Is ammonium nitrate used in drugs?
The most commonly used fertilizer in the drug manufacturing process is anhydrous ammonia, but a drug manufacturer who's a particularly good chemist can also use urea, ammonium nitrate, liquid UAN solutions and other sources of nitrogen.
Is ammonium nitrate safe for humans?
Under normal handling conditions, ammonium nitrate is not harmful. However, inhalation of high concentrations of ammonium nitrate dust can cause respiratory tract irritation. Symptoms may include: coughing, sore throat, shortness of breath, or even suffocation.
What two chemicals will explode when mixed?
For decades, science enthusiasts have delighted at the famously energetic way sodium and potassium explode on contact with water.
Is ammonium nitrate used in bullets?
Ammonium nitrate has been used as an ingredient for conventional ammunition since the beginning of the 20th century. It is mixed with high explosive substances and used to provide additional oxygen during detonation.
What is fertilizer used for illegally?
fertilizers are an appealing chemical to producers ofmethamphetamine and illegal explosives. In part this stems from the proficient results that ammonium nitrate and anhydrous ammonia provide to drug production and domestic terrorism.
Why ammonium nitrate is so explosive?
Over time, the compound absorbs moisture, which can make the beads stick together into a huge rock, says Sella. When such a large quantity of compacted ammonium nitrate is exposed to intense heat — if, say, an accidental fire breaks out — it can trigger an explosion.
Why do farmers spray ammonia on fields?
In addition to its use as a nitrogen fertilizer, anhydrous ammonia has other purposes on the farm. It has been used with high-moisture grains to control mold growth. When using it with grain, use the same precautions that you use when applying it as fertilizer.
Where do you get ammonium nitrate?
You can buy ammonium nitrate as a pure chemical or you can collect it from instant cold packs or some fertilizers.
What happens if ammonium nitrate catches fire?
Although it is not technically classified as an explosive or flammable material, under certain conditions, ammonium nitrate can present a significant explosive threat because it is an oxidizer — an oxygen-rich compound that can accelerate fires or explosions.
Why ammonium nitrate is not used?
Ammonium nitrate is not used in the preparation of ammonia as it is explosive in nature and it decomposes forming nitrous oxide and water vapours.
Do airbags still use ammonium nitrate?
Redesign comes after 6 deaths and hundreds of injuries linked to defective airbags. Less than two weeks after doubling its nationwide recall to 34 million vehicles, Takata has informed federal regulators it will no longer use ammonium nitrate in the design of its airbag inflators.
How is ammonium nitrate used in the Westing Game?
It's used in fertilizers, explosives, and rocket propellants. How is ammonium nitrate used? What assignment does Theo give to Doug Hoo? All the heirs have to be at the Westing house on Saturday night.
What chemicals are used in explosives?
Examples of explosive and potentially explosive chemicals include:Compounds containing the functional groups azide, acetylide, diazo, nitroso, haloamine, peroxide, and ozonide.Nitrocellulose.Di- and Tri-nitro compounds.Peroxide forming compounds.Picric acid (dry)2,4-Dinitrophenylhydrazine (dry)Benzoyl peroxide (dry)
What happens when you mix ammonium nitrate and water?
What happens when you mix water and ammonium nitrate? It feels cold when ammonium nitrate is dissolved in water which indicates an endothermic reaction. In an endothermic reaction, the ammonium nitrate dissolves in water, a chemical reaction that absorbs heat rather than releases it.
Where is ammonium nitrate found?
Ammonium nitrate is found as the natural mineral gwihabaite (formerly known as nitrammite) – the ammonium analogue of saltpetre (mineralogial name: niter) – in the driest regions of the Atacama Desert in Chile, often as a crust on the ground or in conjunction with other nitrate, iodate, and halide minerals.
How does ammonium nitrate decompose?
However, it can be induced to decompose explosively by detonation. Large stockpiles of the material can also be a major fire risk due to their supporting oxidation, a situation which can easily escalate to detonation. Explosions are not uncommon: relatively minor incidents occur most years, and several large and devastating explosions have also occurred. Examples include the Oppau explosion of 1921 (one of the largest artificial non-nuclear explosions ), the Texas City disaster of 1947, the 2015 Tianjin explosions in China, and the 2020 Beirut explosion.
What is the effect of ammonium nitrate on rocket propellant?
One practical consequence of this is that ammonium nitrate formed as solid rocket motor propellant develops cracks, leading to the development of phase stabilized ammonium nitrate (PSAN), which incorporates metal halides as stabilisers.
What is the process of oxidizing ammonia?
Ammonia produced by the Haber process can be oxidized to nitric acid by the Ostwald process. Another production method is a variant of the nitrophosphate process : The products, calcium carbonate and ammonium nitrate, may be separately purified or sold combined as calcium ammonium nitrate .
How much humidity does ammonium nitrate have?
Ammonium nitrate has a critical relative humidity of 59.4%, above which it will absorb moisture from the atmosphere. Therefore, it is important to store ammonium nitrate in a tightly sealed container. Otherwise, it can coalesce into a large, solid mass. Ammonium nitrate can absorb enough moisture to liquefy.
Why is ammonium nitrate being phased out?
Many countries are phasing out its use in consumer applications due to concerns over its potential for misuse. Accidental ammonium nitrate explosions have killed thousands of people since the early 20th century.
What is anfo used for?
It is used in coal mining, quarrying, metal mining, and civil construction in undemanding applications where the advantages of ANFO's low cost and ease of use matter more than the benefits offered by conventional industrial explosives, such as water resistance, oxygen balance, high detonation velocity, and performance in small diameters.
What is ammonium nitrate and what is it used for?
A HUGE explosion erupted in Beirut on August 4 causing 100 deaths and nearly 4,000 injuries.
Inside the space cemetery where the ISS will crash & be buried with old rockets
Most countries have regulations controlling the storage of ammonium nitrate to make it safe.
Why is ammonium nitrate used in dynamite?
Because solid ammonium nitrate can undergo explosive decomposition when heated in a confined space, government regulations have been imposed on its shipment and storage . After the straight dynamites and gelatins, the next important advance in dynamite was the substitution of ammonium nitrate for part of the...
What is NH4NO3 used for?
ammonium nitrate, (NH4NO3), a saltof ammoniaand nitric acid, used widely in fertilizers and explosives. The commercial grade contains about 33.5 percent nitrogen, all of which is in forms utilizable by plants; it is the most common nitrogenous component of artificial fertilizers. Ammonium nitratealso is employed to modify the detonation rate of other explosives, such as nitroglycerinin the so-called ammonia dynamites, or as an oxidizing agent in the ammonals, which are mixtures of ammonium nitrate and powdered aluminum.
What is the most common nitrogen in fertilizer?
The commercial grade contains about 33.5 percent nitrogen, all of which is in forms utilizable by plants; it is the most common nitrogenous component of artificial fertilizers. Ammonium nitrate also is employed to modify the detonation rate of other explosives, such as nitroglycerin in the so-called ammonia dynamites, ...
What is the most common nitrogenous component of artificial fertilizers?
Ammonium nitrate, a salt of ammonia and nitric acid, used widely in fertilizers and explosives. The commercial grade contains about 33.5 percent nitrogen, all of which is in forms utilizable by plants; it is the most common nitrogenous component of artificial fertilizers.
Is ammonium nitrate a crystalline substance?
Ammonium nitrate is a colourless crystalline substance (melting point 169.6 °C [337.3 °F]). It is highly soluble in water; heating of the water solution decomposes the salt to nitrous oxide(laughing gas). Because solid ammonium nitrate can undergo explosive decomposition when heated in a confined space, government regulations have been imposed on its shipment and storage.
Why is ammonium nitrate used in agriculture?
It is actually sold in the form of pellets that are coated with clay. The reason why it is very popular in agriculture is because of the high nitrogen amount in this compound. Nitrogen is a very important plant nutrient that assists in the growth and metabolic processes that the plant undergoes.
What is the chemical binding of ammonium nitrate?
Ammoinium nitrate chemical bindings and formula. Ammonium nitrate was said to be developed Germans which they used as fertilizers instead of Chilean Nitrates since it is a lot cheaper. Commercially, it is prepared by mixing nitric acid and ammonia salt. The reaction from the two substances combined will form Ammonium Nitrate.
What is the name of the compound that is made of nitric acid and salt of ammonia?
Interesting Facts about Ammonium Nitrate. Ammonium nitrate is a chemical compound with the formula NH4NO3. It is composed of nitric acid and salt of ammonia. In room temperature, ammonium nitrate appears in a white crystalline form and it is also colorless.
Why is ammonium nitrate paired with TNT?
It is a strong oxidizing agent. This means that it can actually remove certain electrons from other reactants when subjected to a redox chemical reaction. This is the reason why ammonium nitrates are paired and added in combustibles like TNT and others.
Why is nitrogen important for plants?
Nitrogen is a very important plant nutrient that assists in the growth and metabolic processes that the plant undergoes . Agriculturists love using ammonium nitrate since it is a cheap alternative to expensive fertilizers. It can also yield rapid growth and may increase the fruit production capacity of a plant.
Does ammonium nitrate affect the quality of green leafy vegetables?
It can also yield rapid growth and may increase the fruit production capacity of a plant. It may also affect the quality of green leafy vegetables since the nitrogen which is used by the plants is actually very helpful in the process of photosynthesis. Another famous use of ammonium nitrate is as an additive in explosives.
Is ammonium nitrate regulated?
Ammonium nitrates are now regulated by the government since it is already used to create fertilizer bombs. These are improvised explosive devices that other people use in terrorism. Ammonium nitrate can be very helpful in agriculture but correct storage and handling should always be observed.
What is ammonium nitrate?
CAMEO Chemicals. Ammonium Nitrate Emulsion, Suspension, or Gel is ammonium nitrate suspended in a liquid. The material itself does not readily burn but will readily do so if contaminated by combustible material.
What is the Ammonium Nitrate Security Program?
Ammonium Nitrate Security Program; Proposed Rule: This proposed rule would implement anti-terrorism measures to better secure the homeland. The Department of Homeland Security would regulate the sale and transfer of ammonium nitrate pursuant to section 563 of the Fiscal Year 2008 Department of Homeland Security Appropriations Act with the purpose of preventing the use of ammonium nitrate in an act of terrorism. This proposed rule seeks comment on both proposed text for such a regulation and on several practical and legal issues integral to the development of an Ammonium Nitrate Security Program.
How to prevent ammonium nitrate explosion?
Actions that may help to prevent explosions include: (1) Avoid heating ammonium nitrate in a confined space (e.g., processes involving ammonium nitrate should be designed to avoid this possibility). (2) Avoid localized heating of ammonium nitrate, potentially leading to development of high temperature areas. (3) Ensure that ammonium nitrate is not exposed to strong shock waves from explosives. (4) Avoid contamination of ammonium nitrate with combustible materials or organic substances such as oils and waxes. (5) Avoid contamination of ammonium nitrate with inorganic materials that may contribute to its sensitivity to explosion, including chlorides and some metals, such as chromium, copper, cobalt, and nickel. (6) Maintain the pH of ammonium nitrate solutions within the safe operating range of the process. In particular, avoid low pH (acidic) conditions.
How much ammonium nitrate is in a solid?
Approximately 60 percent of the ammonium nitrate produced in the U. S. is sold as a solid product. To produce a solid product, the ammonium nitrate solution is concentrated in an evaporator or concentrator. The resulting "melt" contains about 95 to 99.8 percent ammonium nitrate at approximately 149 °C (300 °F).
How is ammonium nitrate produced?
Ammonium nitrate (NH4NO3) is produced by neutralizing nitric acid (HNO3) with ammonia (NH3) . ... All ammonium nitrate plants produce an aqueous ammonium nitrate solution through the reaction of ammonia and nitric acid in a neutralizer.
How many people are exposed to ammonium nitrate?
According to the 2006 TSCA Inventory Update Reporting data, the number of persons reasonably likely to be exposed in the industrial manufacturing, processing, and use of ammonium nitrate is 1000 or greater; the data may be greatly underestimated (1).
How much nitrogen is lost from fertilizer?
The total immediate loss of nitrous oxide - nitrogen after application of mineral fertilizer is estimated to be 0.004-1.2 teragram/yr.
What Is Ammonium Nitrate?
Farming is one of the biggest industries in the world and requires extremely high quantities of fertilizer. The main purpose of fertilizers is to provide nutrients to the soil and assist the growth of crops.
Why is ammonium nitrate used in fertilizer?
Another reason, and advantage, for which ammonium nitrate is used as a component for fertilizers, as opposed to urea, is that it does not lose nitrogen to the atmosphere .
What temperature does ammonium nitrate decompose?
Ammonium nitrate has a high melting point. However, the temperature needs to reach 200 Celsius for this compound to decompose. This reaction yields nitrous oxide and water in its gaseous form. The reaction can be summarized as follows: NH 4 NO 3 -> N 2 O + 2H 2 O. However, this reaction can also yield nitrogen when rapidly heated.
What is the chemical formula for ammonium nitrate?
This acid-base reaction, summarized by the formula NH 3 + NO 3 -> NH 4 NO 3, has several properties that make it a useful component in fertilizers, explosives, and ice packs. Some of these properties include:
What is the molecular weight of ammonium nitrate?
Ammonium nitrate is composed of nitrogen (N), oxygen (O), and hydrogen (H). When the molecular weight of all the elements in ammonium nitrate is combined, its molecular mass is 80.044 g/mol.
What is the main purpose of fertilizer?
The main purpose of fertilizers is to provide nutrients to the soil and assist the growth of crops.
Is ammonium nitrate harmful?
Normally, ammonium nitrate is not harmful to handle. However, it can cause health issues if ingested or inhaled in large quantities. Some of the side effects that can be experienced if ammonium nitrate is ingested or inhaled include:
What is the code for ammonium nitrate?
For ammonium nitrate (USEPA/OPP Pesticide Code: 076101) there are 0 labels match. /SRP: Not registered for current use in the U.S., but approved pesticide uses may change periodically and so federal, state and local authorities must be consulted for currently approved uses./
What is nitrous oxide used for?
In the production of nitrous oxide ... used primarily as as anesthetic and as an aerosol for food products
What is Ammonium Nitrate?
Nitrogen comes in many forms. This major plant nutrient can be taken in by plants through the roots or from the stoma in the leaves and stems. Additional sources of nitrogen are often added to soil and plants in areas without sufficient natural sources of nitrogen.
How is ammonium nitrate fertilizer made?
Ammonium nitrate fertilizer is a simple compound to make. It is created when ammonia gas reacts with nitric acid. The chemical reaction produces a concentrated form of ammonium nitrate, which produces prodigious amounts of heat. As a fertilizer, the compound is applied as granules and fused with ammonium sulfate to minimize the volatile nature of the compound. Anti-caking agents are also added to the fertilizer.
What are the sources of nitrogen in soil?
One of the first solid nitrogen sources produced in a large scale capacity is ammonium nitrate.
What is fertilizer used for?
The most common uses for the fertilizer are in vegetable gardens and in hay and pasture fertilization due to the high nitrogen content.
Is ammonium nitrate explosive?
In addition to its usefulness as a fertilizer, ammonium nitrate is also employed in certain industrial and construction settings. The chemical compound is explosive and useful in mining, demolition activities, and quarry work.
Can ammonium nitrate be used in food preservation?
In most cases, the compound is very stable and can only become explosive in certain conditions. Food preservation is another area that is using ammonium nitrate. The compound makes an excellent cold pack when one bag of water and one bag of the compound are united.
Is ammonium nitrate a stable compound?
Ammonium nitrate in gardens is made stable with other compounds. The fertilizer is an almost instantly useable form of nitrogen due to its porosity and solubility. It provides nitrogen from both ammonia and nitrate. The standard method of application is by broadcast spreading the granules.
Overview
Ammonium nitrate is a chemical compound with the chemical formula NH4NO3. It is a white crystalline salt consisting of ions of ammonium and nitrate. It is highly soluble in water and hygroscopic as a solid, although it does not form hydrates. It is predominantly used in agriculture as a high-nitrogen fertilizer. Global production was estimated at 21.6 million tonnes in 2017.
Occurrence
Ammonium nitrate is found as the natural mineral gwihabaite (formerly known as nitrammite) – the ammonium analogue of saltpetre (mineralogical name: niter) – in the driest regions of the Atacama Desert in Chile, often as a crust on the ground or in conjunction with other nitrate, iodate, and halide minerals. Ammonium nitrate was mined there until the Haber–Bosch process made it possible to synthesize nitrates from atmospheric nitrogen, thus rendering nitrate mining obsolete.
Production, reactions and crystalline phases
The industrial production of ammonium nitrate entails the acid-base reaction of ammonia with nitric acid:
HNO3 + NH3 → NH4NO3
Ammonia is used in its anhydrous form (a gas) and the nitric acid is concentrated. The reaction is violent owing to its highly exothermic nature. After the solution is formed, typically at about 83% …
Applications
Ammonium nitrate is an important fertilizer with NPK rating 34-0-0 (34% nitrogen). It is less concentrated than urea (46-0-0), giving ammonium nitrate a slight transportation disadvantage. Ammonium nitrate's advantage over urea is that it is more stable and does not rapidly lose nitrogen to the atmosphere.
Ammonium nitrate readily forms explosive mixtures with varying properties when combined wit…
Safety, handling, and storage
Numerous safety guidelines are available for storing and handling ammonium nitrate. Health and safety data are shown on the safety data sheets available from suppliers and from various governments.
Pure ammonium nitrate does not burn, but as a strong oxidizer, it supports and accelerates the combustion of organic (and some inorganic) material. It should not be stored near combustible …
Health hazards
Ammonium nitrate is not hazardous to health and is usually used in fertilizer products.
Ammonium nitrate has an LD50 of 2217 mg/kg, which for comparison is about two-thirds that of table salt.
Disasters
Ammonium nitrate decomposes, non-explosively, into the gases nitrous oxide and water vapor when heated. However, it can be induced to decompose explosively by detonation. Large stockpiles of the material can also be a major fire risk due to their supporting oxidation, a situation which can easily escalate to detonation. Explosions are not uncommon: relatively minor incidents occur most years, and several large and devastating explosions have also occurred. Examples include …
See also
• Resource recovery