Facts, Summary & Definition
- An adduct is a product formed by an addition reaction
- Adducts can only be formed from reactants which have multiple bonds, such as alkenes and carbonyl groups
- Adducts can be formed through two types of addition reaction: electrophilic and nucleophilic
- Adducts often form between Lewis acids and Lewis bases
What is an adduct?
Jump to navigation Jump to search. An adduct (from the Latin adductus, "drawn toward" alternatively, a contraction of "addition product") is a product of a direct addition of two or more distinct molecules, resulting in a single reaction product containing all atoms of all components. The resultant is considered a distinct molecular species.
How are adducts formed in organic chemistry?
As mentioned before, adducts are formed through addition reactions. In this type of reaction, the double bond partially breaks when a reacting molecule attacks and adds on. The reaction below shows an addition reaction between ethene and bromine.
What is an adduct ion in chromatography?
The term “adduct ion” is a popular term among liquid chromatography/mass spectrometry (LC/MS) users to describe ions formed by adduction of alkali metal ions to an analyte molecule in positive ion analysis. Therefore, the common protonated molecule or [M+H]+ is also properly called an adduct ion.
What is a covalent adduct?
…is the formation of an adduct in which the two species are joined by a covalent bond; proton transfers are not normally involved. If both the Lewis acid and base are uncharged, the resulting bond is termed semipolar or coordinate, as in the reaction of boron trifluoride with ammonia:
What is adduct reaction?
An adduct is a product formed at the end of an addition reaction. This is a reaction in which two or more molecules react and combine to form one larger product. This type of reaction can only occur between chemical compounds which have multiple bonds – compounds like alkenes (double bonds) and alkynes (triple bonds).
What is a protein adduct?
Protein adducts are covalent. modifications resulting from reactions between electrophiles and nucleophilic sites in proteins, such as. the N-terminus or the amino acid side chains containing sulfhydryl or amine functionalities.
How are adducts formed in mass spectrometry?
Abstract. Adduct formation is a common ionization method in electrospray ionization mass spectrometry (ESI/MS). However, this process is poorly understood and complicated to control. We demonstrate possibilities to control adduct formation via mobile phase additives in ESI positive mode for 17 oxygen and nitrogen bases ...
What are adducts in biology?
In biology, an adduct is a complex that forms when a chemical binds to a biological molecule, such as DNA or protein. Source: GreenFacts. More: DNA adducts are altered forms of DNA that occur as the result of exposure to carcinogens (in the case of smokers these would be the carcinogens present in cigarette smoke).
What is an adduct in mass spectrometry?
An adduct ion is formed from a precursor ion and contains all of the constituent atoms of that ion as well as additional atoms or molecules. Adduct ions are often formed in a mass spectrometer ion source.
What is DNA adduct formation?
In molecular genetics, a DNA adduct is a segment of DNA bound to a cancer-causing chemical. This process could lead to the development of cancerous cells, or carcinogenesis. DNA adducts in scientific experiments are used as biomarkers of exposure.
What is ammonium adduct?
The ammonium adduct ion, [M+NH,]+, is often ob- served in ammonia-mediated chemical ionization mass spectra of oxygen-containing compounds such as al- cohols, ethers, aldehydes, ketones and esters.
What is an adduct?
Facts, Summary & Definition. An adduct is a product formed by an addition reaction. Adducts can only be formed from reactants which have multiple bonds, such as alkenes and carbonyl groups. Adducts can be formed through two types of addition reaction: electrophilic and nucleophilic.
What are adducts made of?
Facts, Summary & Definition 1 An adduct is a product formed by an addition reaction 2 Adducts can only be formed from reactants which have multiple bonds, such as alkenes and carbonyl groups 3 Adducts can be formed through two types of addition reaction: electrophilic and nucleophilic 4 Adducts often form between Lewis acids and Lewis bases
What is the molecule formed by addition reactions?
Adducts are often known to form between Lewis acids and Lewis bases. The molecule formed is called a Lewis acid-base adduct (or a Lewis acid-base complex). As adducts are only formed through addition reactions (that is, without the simultaneous loss of a group), Lewis-acid and Lewis-base reactions cannot be that of the substitution kind.
What is an electrophilic addition?
Electrophilic Addition. An electrophilic addition reaction is a type of reaction in which a bond is broken, and two new bonds are formed. As discussed earlier, the reactant must have a double or triple bond – the electrophile adds to a pi bond in this instance. This is an addition reaction, and so nothing is lost in the process – all ...
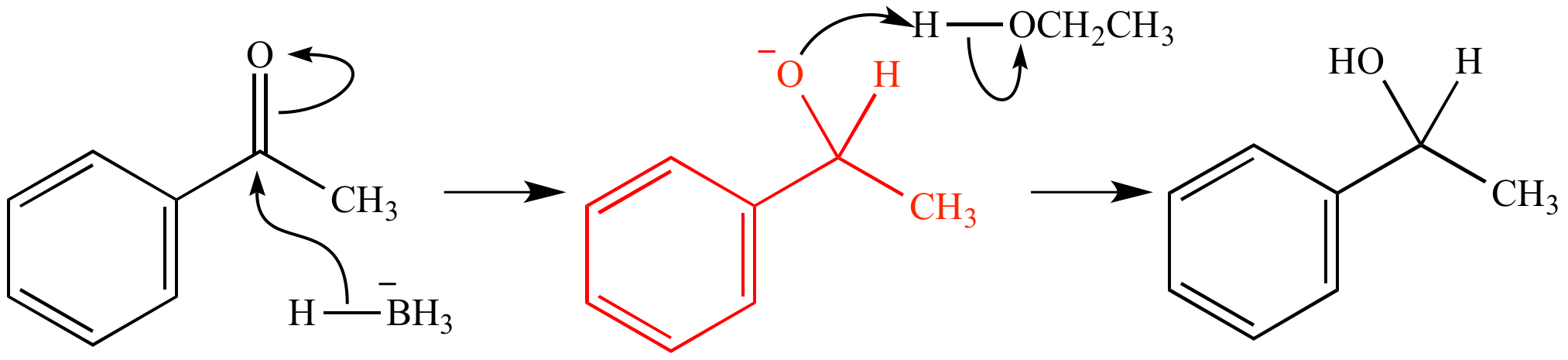
What is the adduct in a reaction between ethene and bromine?
The reaction below shows an addition reaction between ethene and bromine. The adduct in this reaction is 1,2-dibromoethane. This is because it is a distinct species which contains all atoms of all the components.
Where are the atoms in the reactants found in an electrophilic addition reaction?
This is an addition reaction, and so nothing is lost in the process – all of the atoms found in the reactant molecules are found in the adduct, too. The above image shows the basic of an electrophilic addition reaction. As you can see, all of the atoms in the reactants are found in the adduct.
What type of reaction is a double bond?
Nucleophilic Addition. A nucleophilic addition reaction is a type of reaction in which a double bond is broken, and two single bonds are formed. An electron-deficient (electrophilic) double or triple bond reacts with something which is electron-rich (nucleophile). The nucleophile adds to a pi bond in this instance.
What is an adduct?
Definition of adduct (Entry 2 of 2) : a chemical addition product adducts form as carcinogenic metabolites bind to DNA.
What does "adduct" mean?
Definition of adduct. (Entry 1 of 2) transitive verb. : to draw (something, such as a limb) toward or past the median axis of the body also : to bring together (simi lar parts) adduct the fingers.
What does "adduct" mean in medical terms?
Medical Definition of adduct. (Entry 1 of 2) : to draw (as a limb) toward or past the median axis of the body also : to bring together (similar parts) adduct the fingers.
Where does the word "adduce" come from?
borrowed from Medieval Latin adductus, past participle of addūcere "to draw (a limb) toward the body," going back to Latin, "to lead or bring (to a place)" — more at adduce.
What are polyfluoro adducts?
Polyfluoro adducts of o-benzylhydroxylamine, 8, or the hydrochloride have been extensively used to investigate carbonyl groups, in aldehydes (e.g., hexanal) and ketones (e.g., heptanone). Such functional groups have very high volatility and activity and hence it is difficult to quantify such analytes without derivatization. Derivatization of carbonyls with PFBHA can be performed using mild conditions, and the derivatization reaction is very fast (the corresponding oximes form in seconds). 99 Such fluoro-tags make it possible for both GC/ECD and GC/MS or HPLC techniques to be used in many areas of study, concerning the analysis of aldehydes and other carbonyl containing compounds. 100,101
What are PAH adducts?
PAH-DNA adducts provide a reflection of individual variation in exposure and toxicokinetics and have been associated with risk of cancer and reproductive and developmental effects (Tang et al., 2006; Perera et al., 2007 ). In the cohorts followed by researchers from the Columbia Center for Children’s Environmental Health, mean DNA adduct concentrations in both maternal and fetal cord blood, as well as the proportion of samples with detectable adducts, increased across the populations studied in a manner consistent with the trend in estimated ambient exposure to PAHs ( P < .001, northern Manhattan < WTC < Krakow < Tongliang). Data from these four populations indicate that the developing fetus may have a 10-fold greater susceptibility to DNA damage than mothers ( Perera et al., 2005a ).
Can adducts be polymerized?
Adducts can be formed with high and low molecular weight molecules, in particular monomers, and polymerization carried out inside inclusion compounds is a particular case of polymerization in organized systems.
What is the compound 13 used for?
in 2006, compound 13 was extensively used for the synthesis of novel low-valent silicon compounds. For example, the isolation of bis (silylene)s, 29–31 silyliumylidene cation 31, or a diphosphacyclobutadiene 32,33 have been reported. Consequently, also the stabilization of the highly reactive Si (II)-H moiety by the amidinate ligand was investigated. The first attempts were reported by the group of So, which reacted the amidinate-stabilized dichlorosilane 14 with two equivalents of potassium graphite to yield the silylsilylene PhC (N t Bu) 2 Si- [Si (N t Bu) 2 -CHPh]Cl 15 ( Scheme 6 ). The formation of the chlorosilylene and hydrosilylene intermediate was suggested followed by a reaction of the PhC (N t Bu) 2 SiH intermediate with the aminidate ligand of the chlorosilylene intermediate 13 giving silylsilylene 15. The reaction of the chlorosilylene 13 with K [HB ( i Bu) 3] led to the same product. This result confirms the formation of the suggested intermediate PhC (N t Bu) 2 SiH in solution, which further reacts with the chlorosilylene 13. 34
Which ligand stabilizes alkyl complexes?
Several alkyl complexes stabilized by both alkoxides and imido ligands are described in Section Alkyl, alkylidene, and alkylidyne complexes stabilized by imido, amido and other anionic N-donor ligands devoted to Alkyl complexes supported by imido ligands.