
al·ka·li
- A carbonate or hydroxide of an alkali metal, the aqueous solution of which is bitter, slippery, caustic, and characteristically basic in reactions.
- Any of various soluble mineral salts found in natural water and arid soils.
- Alkali metal.
- A substance having highly basic properties; a strong base.
What does alkali feel like?
Like acids, their bottles are labelled with a symbol to warn that they may make your skin red or blistered unless you wash off any spills with plenty of water. Alkalis feel soapy when they get on your skin, so it is easy to tell when you have had an accident and must wash your hands. Just like concentrated acids, concentrated alkalis are corrosive.
What is an everyday example of an alkali?
- Vinegar is diluted acetic acid, which is what gives salad dressings and pickled vegetables their tart taste.
- Oranges, lemons, and limes contain citric acid, which gives them their sour taste
- Wine contains tartaric acid
- Aspirin contains acetylsalicylic acid
What are 4 examples of alkalis?
Uses of common Alkalis
- Sodium hydroxide is used to make paper, detergents and soap.
- Potassium hydroxide is used by farmers to make acidic soil more alkaline so that plants will grow better in it, and is also used as the electrolyte in alkaline, Ni-Cd, ...
- Calcium hydroxide is used to neutralize acidic soil.
- Ammonium hydroxide is used as a cleansing agent.
What are the uses of alkali?
What are the Uses of Alkalis? 1) Sodium hydroxide is used in the manufacture. of paper, soap and ceramics. Ceramics include plates , cups, bricks and tiles. Sodium hydroxide is used as an alkali cleaner and. to treat aluminium before anodising. Sodium. hydroxide is also used to neutralise acids to make salts.
What is alkali in one word answer?
The definition of an alkali is a soluble salt that comes from the ashes of plants and is made up of mostly potassium or sodium carbonate. Lye and calcium carbonate are each an example of an alkali. Soda ash; caustic soda, caustic potash, etc. Any of various soluble mineral salts found in natural water and arid soils.
How do you explain alkaline to a child?
Any substance that has a pH higher than 7 is an alkali (or alkaline) substance, which is also called basic. Any substance that has a pH lower than 7 is an acid.
What is the difference acid and alkali?
Acids are a group of chemicals that contain a H= ion example's of which are vinegar, Hydrochloric acid and Sulphuric acid. Alkalis are a group of chemicals that contain the OH= ion and have a soapy feel. An example is Sodium Hydroxide. In solid form they are called bases and in solution alkalis.
What is difference between alkali and base?
The difference between an alkali and a base is: Alkali compounds are types of bases that dissolve in water whereas the base neutralizes the acid. All alkali are bases but all bases are not alkalis. Alkali is used for metals of group 1 in the periodic table whereas the base is a compound having OH ions.
What is alkaline in human body?
Alkalinity means that something has a pH higher than 7. The human body is naturally slightly alkaline , with a blood pH of around 7.4. The stomach is acidic, which allows it to digest food. The pH of saliva and urine changes depending on diet, metabolism, and other factors.
What taste is alkaline?
There is no single word to describe that taste, and no single taste receptor for alkaline substances either (whereas there are taste receptors for acids). The most recognizable taste to encounter would be "soapy".
What do alkalis feel like?
Alkalis feel soapy when they get on your skin, so it is easy to tell when you have had an accident and must wash your hands. Just like concentrated acids, concentrated alkalis are corrosive. They can attack metals and destroy skin if spilled, so their containers are labelled with a warning symbol.
Is alkaline water good for kids?
Published by Peak Alkalinity on February 8, 2019. If you have been wondering if alkaline water is safe for your child, the answer is yes! Children can drink alkaline water safely while also experiencing its many benefits, just as adults do.
What is an alkali solution?
Alkali. In chemistry, an alkali is an aqueous (from water) solution with a pH value of more than 7. The word 'Alkali' comes from the Arabic 'qali' meaning 'from the ashes' since ashes mixed with water used as cleaning products (such as soaps) are made of alkali materials. An alkali is where a base is dissolved in water.
How to find the strength of an alkali?
The strength of an alkali can be found using universal indicator. Also like acids, the strength of an alkali is rated using the pH scale. For example, soap and toothpaste contain weak alkalis, while cleaning products often contain strong ones. Sodium Hydroxide.
Why is potassium hydroxide used in plants?
Potassium hydroxide is used by farmers to make acidic soil more alkaline so that plants will grow better in it, and is also used as the electrolyte in alkaline, Ni-Cd, and Ni-MH batteries.
What is ammonium hydroxide used for?
Ammonium hydroxide is used as a cleansing agent.
Is alkali corrosive or corrosive?
It is corrosive (it can burn your skin away) The higher the number is over 7 on the pH scale the stronger the alkali is. Highly soluble (can be dissolved) in water. They have a bitter taste. Turns red litmus paper blue. Can conduct electricity due to the presence of mobile ions. Is blue or purple on universal indicator.
What is the opposite of alkali?
The opposite of an alkali is an acid. Instead of a bitter taste, acids tend to have a sour taste. Things such as lemons and vinegar are acids, or acidic.
What is the pH scale?
The pH scale measures whether a substance is an alkali or an acid and ranges from 0-14, with 7 being neutral. When a substance has more hydrogen atoms (H+), it is an acid (below 7 pH), and when a substance has more hydroxide atoms (OH-), it is an alkali (above 7 pH). To unlock this lesson you must be a Study.com Member.
How does pH work?
The pH of a substance depends on how atoms are arranged and combined in the substance. Atoms are the smallest unit and make up every object and substance in the universe. Remember that pure water is right in the middle of the scale and has a pH of 7.
What is the pH of distilled water?
Pure, distilled water has a pH of 7. This is called neutral and is right in the middle of the scale. Any substance that has a pH higher than 7 is an alkali (or alkaline) substance, which is also called basic. Any substance that has a pH lower than 7 is an acid. Lesson.
Is toothpaste alkali or neutral?
Toothpaste is also alkali and is what helps keep bacteria off your teeth and keep those guys pearly white. Your blood has a pH of 7.4, which is close to neutral. If you ingest cleaners or even your toothpaste, this could make you very sick and in need of medical care immediately.
Is a household cleaner an acid or a base?
This means that it contains an equal amount of hydrogen atoms (H+) and hydroxide atoms (OH-). When a substance has more hydrogen atoms (H+), it is an acid. When a substance has more hydroxide atoms (OH-), it is an alkali, or basic. Many household cleaners are alkali.
What is an alkali?
In chemistry, an alkali ( / ˈælkəlaɪ /; from Arabic: القلوي al-qaly "ashes of the saltwort ") is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water.
What is the origin of alkali?
The word "alkali" is derived from Arabic al qalīy (or alkali ), meaning the calcined ashes (see calcination ), referring to the original source of alkaline substances. A water-extract of burned plant ashes, called potash and composed mostly of potassium carbonate, was mildly basic. After heating this substance with calcium hydroxide ( slaked lime ), a far more strongly basic substance known as caustic potash ( potassium hydroxide) was produced. Caustic potash was traditionally used in conjunction with animal fats to produce soft soaps, one of the caustic processes that rendered soaps from fats in the process of saponification, one known since antiquity. Plant potash lent the name to the element potassium, which was first derived from caustic potash, and also gave potassium its chemical symbol K (from the German name Kalium), which ultimately derived from al k ali.
What are the properties of alkali and bases?
Common properties of alkalis and bases. Alkalis are all Arrhenius bases, ones which form hydroxide ions (OH −) when dissolved in water. Common properties of alkaline aqueous solutions include: Moderately concentrated solutions (over 10 −3 M) have a pH of 7.1 or greater.
What are the properties of alkaline aqueous solutions?
Alkalis are all Arrhenius bases, ones which form hydroxide ions (OH −) when dissolved in water. Common properties of alkaline aqueous solutions include: 1 Moderately concentrated solutions (over 10 −3 M) have a pH of 7.1 or greater. This means that they will turn phenolphthalein from colorless to pink. 2 Concentrated solutions are caustic (causing chemical burns). 3 Alkaline solutions are slippery or soapy to the touch, due to the saponification of the fatty substances on the surface of the skin. 4 Alkalis are normally water-soluble, although some like barium carbonate are only soluble when reacting with an acidic aqueous solution.
What is the pH of a soil?
Soils with pH values that are higher than 7.3 are usually defined as being alkaline. These soils can occur naturally, due to the presence of alkali salts. Although many plants do prefer slightly basic soil (including vegetables like cabbage and fodder like buffalo grass ), most plants prefer a mildly acidic soil (with pHs between 6.0 and 6.8), and alkaline soils can cause problems.
What is a basic salt?
A basic salt of an alkali metal or alkaline earth metal (This includes Mg (OH) 2 ( magnesium hydroxide) but excludes NH 3 ( ammonia ).) Any base that is soluble in water and forms hydroxide ions or the solution of a base in water. (This includes both Mg (OH) 2 and NH 3 .)
Is alkali a base?
The terms "base" and "alkali" are often used interchangeably, particularly outside the context of chemistry and chemical engineering .
Where does the word "alkali" come from?
Both alkali ne and alkali come from the Arabic word “al-qili.”
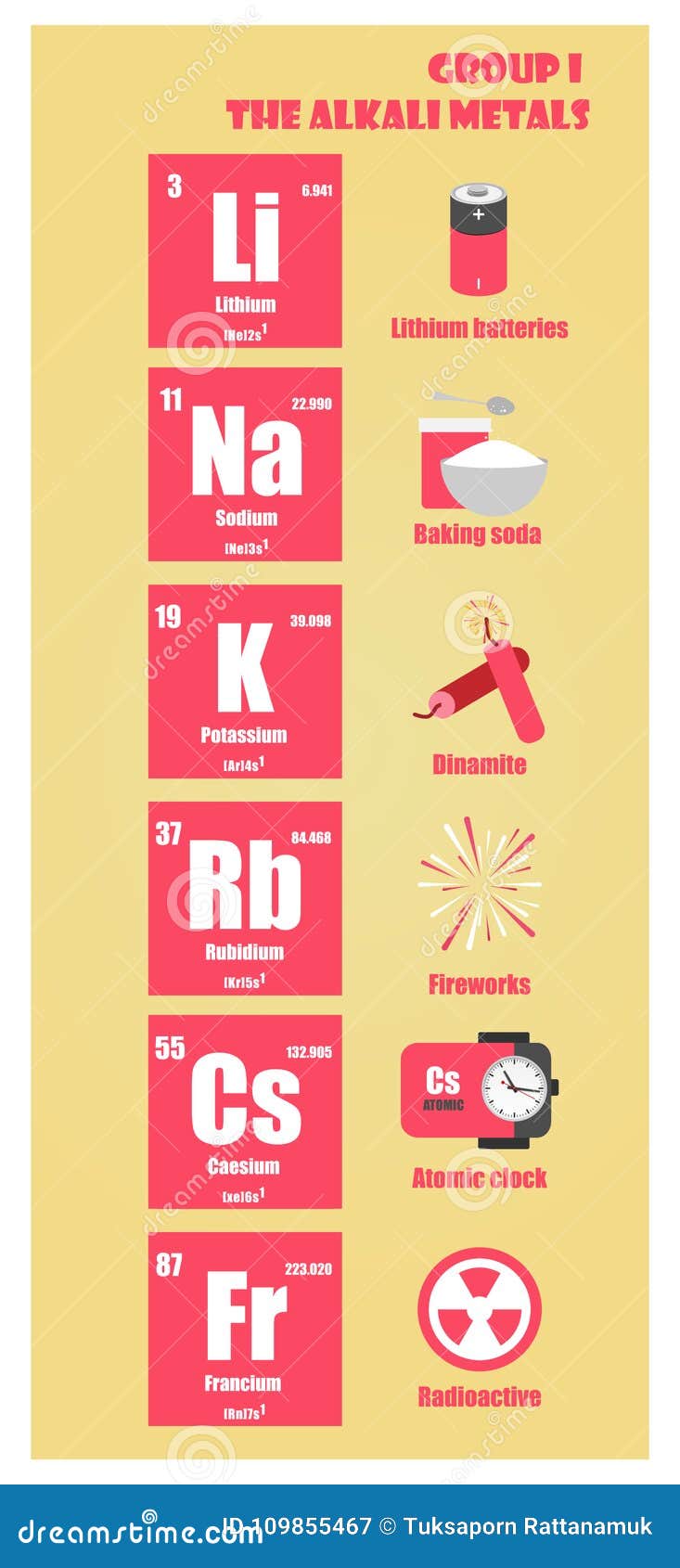
What is an alkali metal?
alkali. A carbonate or hydroxide of an alkali metal, the aqueous solution of which is bitter, slippery, caustic, and characteristically basic in reactions. Any of various soluble mineral salts found in natural water and arid soils. Alkali metal.
What is an aqueous solution of alkali?
A hydroxide of an alkali metal. The aqueous solution of alkalis is bitter, slippery, caustic, and characteristically basic in reactions.
Do plants have difficulty growing in soil that is rich in alkalis?
Plants have difficulty growing in soil that is rich in alkalis.
Can cations interfere with alkali ne?
Now, cations other than the alkali ions are liable to interfere with tests designed for alkali ne or neutral solutions.
What does alkali mean?
alkali in British English. (ˈælkəˌlaɪ ) noun Word forms: plural -lis or -lies. 1. chemistry. a soluble base or a solution of a base. 2. a soluble mineral salt that occurs in arid soils and some natural waters. Collins English Dictionary. Copyright © HarperCollins Publishers.
What does alkali mean in science?
1. Chemistry. a. any of various bases, the hydroxides of the alkali metals and of ammonium, that neutralize acids to form salts and turn red litmus paper blue. b. any of various other more or less active bases, as calcium hydroxide.
What is a base or hydroxide?
1. any base or hydroxide, as soda, potash, etc. that is soluble in water and gives a high concentration of hydroxyl ions in solution; specif., any of the hydroxides and carbonates of the alkali metals. 2.
What is the pH of an alkali?
An alkali is a substance with a pH value of more than 7. Alkalis form chemical salts when they are combined with acids .
What is soda ash called?
This product was called soda ash (was also called alkali).
What is a soluble salt?
any of various other compounds, as the carbonates of sodium and potassium. 2. Agriculture. a soluble mineral salt or a mixture of soluble salts , present in some soils, esp. in arid regions, and detrimental to the growing of most crops.
What is the pH of a strong alkali?
any soluble substance, as a mineral salt or mixture of salts, that can neutralize acids, has a pH greater than 7.0, and turns litmus blue: strong alkalies are caustic. Webster’s New World College Dictionary, 4th Edition. Copyright © 2010 by Houghton Mifflin Harcourt. All rights reserved.
What is an alkali metal?
Alkali metals are any of the elements found in Group IA of the periodic table (the first column). Alkali metals are very reactive chemical species that readily lose their one valence electron to form ionic compounds with nonmetals. All elements in the alkali metal group occur in nature.
Why is hydrogen considered an alkali metal?
The alkali metals are: The International Union of Pure and Applied Chemistry (IUPAC) excludes hydrogen (H) as an alkali metal because it occurs as a gas under ordinary temperatures and pressures.
Why are alkali metals stored in an inert atmosphere?
Although they are soft enough to cut with a knife, exposing a bright surface, the metals react with water and air and quickly tarnish, so the pure metals are stored in an inert atmosphere or under oil to prevent oxidation.
Do alkali metals react with water?
All of the metals react vigorously with water, with the energy of the reaction increasing as you move down the periodic table. None of the alkali metals exists free in nature: They are found as salts, forming crystals with the body-centered cubic structure.
What does "alkaline" mean?
Definition of alkaline. : of, relating to , containing, or having the properties of an alkali or alkali metal : basic especially, of a solution : having a pH of more than 7.
What does alkaline mean in medical terms?
Medical Definition of alkaline. : of, relating to, containing, or having the properties of an alkali or alkali metal : basic especially, of a solution : having a pH of more than 7. Other Words from alkaline.

Overview
In chemistry, an alkali is a basic, ionic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The adjective alkaline is commonly, and alkalescent less often, used in English as a synonym for basic, especially for bases soluble in water. This broad use of the term is likely to have come about because alkalis were the first bases known to obey the Arrhenius definition of a …
Etymology
The word "alkali" is derived from Arabic al qalīy (or alkali), meaning the calcined ashes (see calcination), referring to the original source of alkaline substances. A water-extract of burned plant ashes, called potash and composed mostly of potassium carbonate, was mildly basic. After heating this substance with calcium hydroxide (slaked lime), a far more strongly basic substance known as caustic potash (potassium hydroxide) was produced. Caustic potash was traditionally used in co…
Common properties of alkalis and bases
Alkalis are all Arrhenius bases, ones which form hydroxide ions (OH ) when dissolved in water. Common properties of alkaline aqueous solutions include:
• Moderately concentrated solutions (over 10 M) have a pH of 10 or greater. This means that they will turn phenolphthalein from colorless to pink.
• Concentrated solutions are caustic (causing chemical burns).
Difference between alkali and base
The terms "base" and "alkali" are often used interchangeably, particularly outside the context of chemistry and chemical engineering.
There are various more specific definitions for the concept of an alkali. Alkalis are usually defined as a subset of the bases. One of two subsets is commonly chosen.
• A basic salt of an alkali metal or alkaline earth metal (This includes Mg(OH)2 (magnesium hydr…
Alkali salts
Alkali salts are soluble hydroxides of alkali metals and alkaline earth metals, of which common examples are:
• Sodium hydroxide (NaOH) – often called "caustic soda"
• Potassium hydroxide (KOH) – commonly called "caustic potash"
Alkaline soil
Soils with pH values that are higher than 7.3 are usually defined as being alkaline. These soils can occur naturally, due to the presence of alkali salts. Although many plants do prefer slightly basic soil (including vegetables like cabbage and fodder like buffalo grass), most plants prefer a mildly acidic soil (with pHs between 6.0 and 6.8), and alkaline soils can cause problems.
Alkali lakes
In alkali lakes (also called soda lakes), evaporation concentrates the naturally occurring carbonate salts, giving rise to an alkalic and often saline lake.
Examples of alkali lakes:
• Alkali Lake, Lake County, Oregon
• Baldwin Lake, San Bernardino County, California
See also
• Alkali metals
• Alkaline earth metals
• Base (chemistry)