What are the reactions of LDA-generated esters?
21.10: Reactions of LDA-Generated Ester Enolates. Ester enolates can be generated with LDA in THF rapidly and quantitatively. The resulting enolates can undergo carbonyl addition reactions with other esters, aldehydes, ketones or alkylation reactions with alkyl halides or tosylates.
What is a lithium enol ester?
Vinyl acetate is an enol ester that is made on an industrial scale. Lithium enolates adopt aggregated structures akin to other lithium alkoxides. Enolates are electronically related to allyl anions. The anionic charge is delocalized over the oxygen and the two carbon sites.
What are enolates?
Enolates are organic anions derived from the deprotonation of carbonyl compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
How to study enolates derived from β-amino esters?
The solution structures of four enolates derived from β-amino esters are investigated using 6Li NMR spectroscopy in conjunction with the method of continuous variation (method of Job).

What makes something an enolate?
Enolates result from the removal of a proton on the carbon adjacent to a carbonyl (the “alpha carbon”). They are stable due to inductive effects, but more importantly due to the delocalization (via resonance) of the lone pair on carbon to more electronegative oxygen.
What is enolate in chemistry?
Enolate ions are derivatives of ketones and aldehydes (compounds containing a double bond between carbon and oxygen atoms), from which they can be generated by abstraction of a proton from the carbon atom that is located next to the carbon of the carbonyl group.
Can esters form enols?
As we mentioned, esters can also form enolates, though in smaller concentrations than aldehydes or ketones. These enolates can then react with unreacted starting material in a condensation reaction that is exactly analogous to the aldol reaction.
Is carboxylic acid An enolate?
Ketones, carboxylic esters, carboxylic acids, and carboxamides can in general be converted to enolates by deprotonation with strong bases like lithium diisopropylamide. The enolates can also be formed by conjugate reduction reactions.
What are enolates give two examples?
There are only two types of enolates: those with the metal closer to the oxygen atom and those with the metal closer to the carbon atom. Enolates from Groups I, II, and III exist as O-metal tautomers. These highly electropositive metals form a strong bond with the oxygen atom.
How do you identify enolate?
Identifying Enolates : Example Question #1 Enolates are formed by an oxygen anion bound to an alkene carbon. Reactions II and III include an enolate intermediate, as shown in the mechanisms below, whereas reaction I is a simple SN2 reaction and does not include an enolate intermediate. Enolates are highlighted in red.
What is the difference between enol and enolate?
The main difference between enols, enolates, and enamines is that enols have a hydroxyl group next to a C=C double bond, whereas enolates have a negative charge on the oxygen atom of an enol and enamines have an amine group next to a C=C double bond.
What Cannot form an enolate?
q Since carbonyl compounds which do not have alpha hydrogens can not form an enolate, they cannot undergo the aldol reaction.
Are enols and enolates the same?
Enols are formed by the removal of a hydrogen atom from the alpha carbon of a carbonyl compound. Enolates are formed from enols using a base; the base can react with the hydrogen atom of the hydroxyl group of Enol. Enamines are formed by the condensation of an aldehyde or ketone with a secondary amine.
How do you convert carboxylic acid to ester?
Esters are produced when carboxylic acids are heated with alcohols in the presence of an acid catalyst. The catalyst is usually concentrated sulphuric acid. Dry hydrogen chloride gas is used in some cases, but these tend to involve aromatic esters (ones containing a benzene ring).
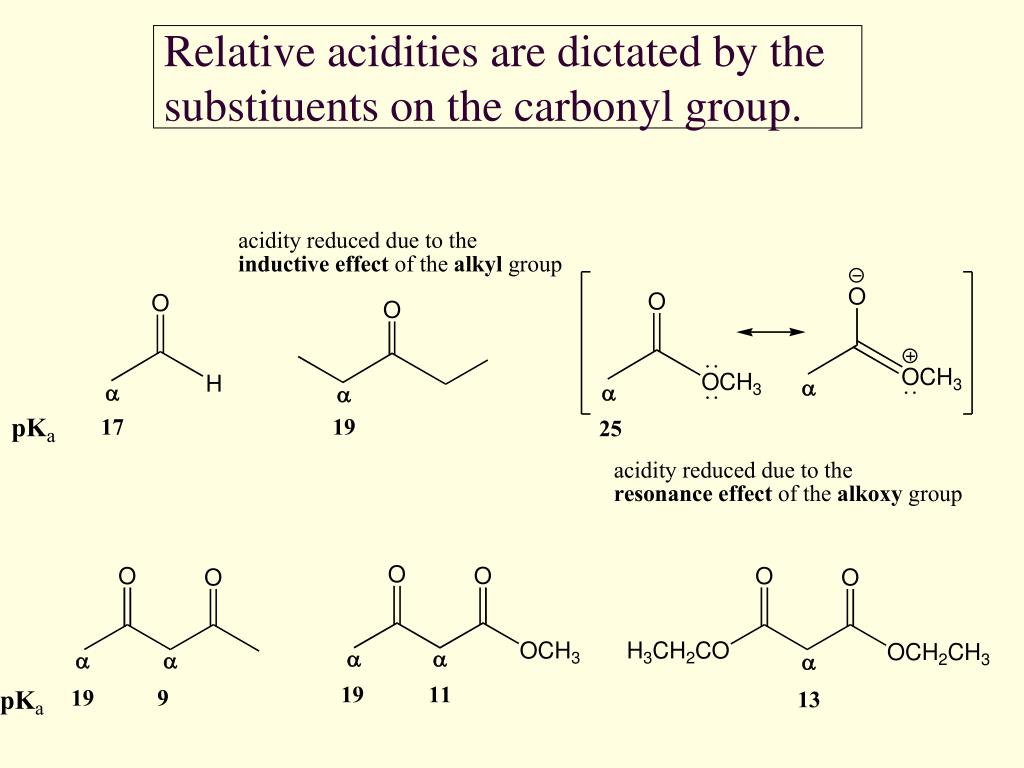
Are esters more acidic than aldehydes?
In the ester, there is also a resonance donation from the alkoxy group towards the carbonyl that competes with the stabilisation of the enolate charge. This makes the ester enolate less stable than those of aldehydes and ketones so esters are even less acidic.
Do ketones react with enolate ions?
Enolate ions can also react with aldehydes and ketones by nucleophilic addition. The enolate ion acts as the nucleophile while the aldehyde or ketone acts as an electrophile.
What is ENOL and enolate?
Enols are alkenes with a hydroxyl group attached to one of the double bonds' carbon atoms. Enedials are alkenes containing hydroxyl groups on both sides of the double bond. Enolates are the deprotonated anions of enols. A reductone is a chemical having an enediol structure and a carbonyl group next to it.
What is the difference between an enol and enolate?
Enols are formed by the removal of a hydrogen atom from the alpha carbon of a carbonyl compound. Enolates are formed from enols using a base; the base can react with the hydrogen atom of the hydroxyl group of Enol. Enamines are formed by the condensation of an aldehyde or ketone with a secondary amine.
Is an enolate a nucleophile?
Enamine, enolates and enols are all turbo-charged nucleophiles. The nucleophilic atom is the alpha carbon.
What is a imine in chemistry?
An imine is a functional group or chemical compound containing a carbon–nitrogen double bond. Imines are chemical molecules with a double bond between carbon and nitrogen (C=N). They are made by substituting the oxygen atom in aldehydes and ketones with the (N-R) group. Imines are compounds with a C=N double bond.
What are enolates used for?
Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
How does aza enolate alkylate?
Alkylation of aza enolates via epoxide ring opening of oxetane. Since epoxide is a three-membered ring molecule, is has a high degree of ring strain. Although the carbons in the ring system are tetrahedral, preferring 109.5 degrees between each atom, epoxide strains the ring angles into 60 degrees.
How is aza enolate formed?
Aza enolates can also be formed with Grignard reagents and react with other soft electrophiles, including Michael receptors.
How are enolates trapped?
Enolates can be trapped by acylation and silylation, which occur at oxygen . Silyl enol ethers are common reagents in organic synthesis as illustrated by the Mukaiyama aldol reaction:
What is the deprotonation of enolizable ketones?
Deprotonation of enolizable ketones, aldehydes, and esters gives enolates. With strong bases, the deprotonation is quantitative. Typically enolates are generated from using lithium diisopropylamide (LDA).
What are the factors that affect the behavior of enolates?
Many factors affect the behavior of enolates, especially the solvent, additives (e.g . diamines), and the countercation (Li + vs Na +, etc.). For unsymmetrical ketones, methods exist to control the regiochemistry of the deprotonation. Deprotonation using LDA. The deprotonation of carbon acids can proceed with either kinetic or thermodynamic reaction ...
Which type of bond has less electronegative nitrogen?
Therefore, with more electrophilic carbon, aldehydes allow for better nucleophilic addition to the carbon on the carbon-oxygen double bond. On the other hand, imine has less electronegative nitrogen which induces a weaker partially positive charge on the carbonyl-carbon.
What are the resonances of benzyl enolate 7in?
The corresponding analysis of benzyl-substituted enolate 7in 9.0 M THF at -25 °C revealed only three resonances, consistent with either an ensemble of tetramers (R4/S4, R3S1/R1S3, and R2S2) or an ensemble of hexamers in which two resonances are unresolved. Failure of the tetramer-based model was suggested by a poor parametric fit (Figure 3). By contrast, the fit to a hexamer model assuming the two resonances corresponding to the R3/S3and R4S2/R2S4hexamers are superimposed afforded a distinct improvement (Figure 4). The latter fit is further justified by the sporadic appearance of the fourth resonance as a shoulder. A similar problem surfaced during the analysis of R/S mixtures of phenyl-substituted enolate 8in which resonances corresponding to R6and R5S1/R1S5do not resolve in 9.0 M THF. In the case of 8, however, all four 6Li resonances fully resolve in 3.0 M THF/toluene, affording a Job plot of comparable quality to that in Figure 1consistent with hexamers (Supporting Information).
How many hexamers are in a R/R mixture?
Mixtures of structurally distinct enolates derived from the same stereochemical series (R/R’ mixtures) afford an ensemble of seven spectroscopically distinct hexamers. Each of the RmR’nhexamers should mimic the optically pure (R6) forms. Success depended critically on the choice of enolates.
Why are Li and O omitted?
Li and O have been omitted for clarity and N = NH2.

Overview
Enolates are organic anions derived from the deprotonation of carbonyl compounds. Rarely isolated, they are widely used as reagents in the synthesis of organic compounds.
Bonding and structure
Enolate anions are electronically related to allyl anions. The anionic charge is delocalized over the oxygen and the two carbon sites. Thus they have the character of both an alkoxide and a carbanion.
Although they are often drawn as being simple salts, in fact they adopt complicated structures often featuring aggregates.
Preparation
Deprotonation of enolizable ketones, aldehydes, and esters gives enolates. With strong bases, the deprotonation is quantitative. Typically enolates are generated from using lithium diisopropylamide (LDA).
Often, as in conventional Claisen condensations, Mannich reactions, and aldol condensations, enolates are generated in low concentrations with alkoxide ba…
Reactions
As powerful nucleophiles, enolates react readily with a variety of electrophiles. These reactions generate new C-C bonds and often new stereocenters. The stereoselectivity and regioselectivity is influenced by additives, solvent, counterions, etc. One important class of electrophiles are alkyl halides, and in this case a classic problem arises: O-alkylation vs C-alkylation. Controlling thi…
Aza enolates
Aza enolates (also known as imine anions, enamides, metallated Schiff bases, and metalloenamines) are nitrogen analogous to enolates. When imines get treated with strong bases such as LDA, highly nucleophilic aza enolates are generated.
The major benefit of using aza enolates is that they don't undergo self-condens…
See also
• Nitrile anion