What does it mean to have a neutral pH?
Mar 01, 2020 · Neutral solutions maintain a pH of 7. Water and human blood are great examples of neutral solutions. Acids mixed with bases can be neutralized and given a pH of 7. Water can break down to form (H+) and (OH-) ions. When these ions are equal to each other, the value is 1 *10^-7, or neutral. Click to see full answer.
What substances have a neutral pH?
Apr 27, 2020 · A pH of 7 is neutral. A pH less than 7 is acidic. Similarly, it is asked, what is the pH of a base? The pH scale is often said to range from 0 to 14, and most solutions do fall within …
How to make your own neutral pH floor cleaners?
Examples of neutral substances. Milk. It is a neutral substance (pH 6.5). However, when it comes into contact with gastric juices it stimulates the decrease in stomach pH so, contrary to what is …
What is pH neutral level?
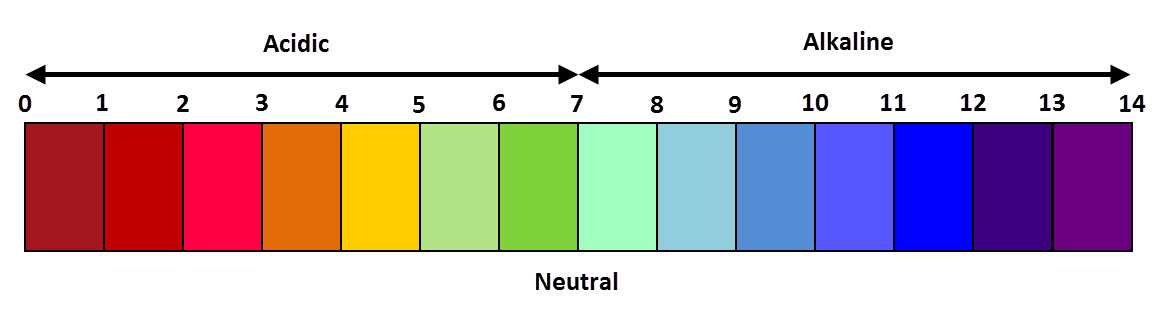
Apr 08, 2014 · pH or potential of hydrogen is a scale of acidity from 0 to 14. It tells how acidic or alkaline a substance is. More acidic solutions have lower pH (less than 7). More alkaline …

What is considered a neutral pH?
The range goes from 0 - 14, with 7 being neutral. pHs of less than 7 indicate acidity, whereas a pH of greater than 7 indicates a base. pH is really a measure of the relative amount of free hydrogen and hydroxyl ions in the water.Jun 19, 2019
Is pH 7.5 neutral?
Knowing the dependence of pH on [H+], we can summarize as follows: If pH < 7, then the solution is acidic. If pH = 7, then the solution is neutral. If pH > 7, then the solution is basic.Apr 10, 2021
What solution has a neutral pH?
This pH value of 7 is important because it indicates a neutral solution. All other substances are compared to this neutral point. Any solution that has a pH of less than 7 is considered acidic, and anything above a pH of 7 is basic.
Is a pH of 7.3 neutral?
The pH Scale While 7 is considered pH neutral, any value below 7 is acidic, and any value above 7 is basic.Apr 30, 2018
Is 7.9 basic or neutral?
The pH scale ranges from 0 to 14. The readings are based around a pH of 7, which is neutral, like pure water: A pH below 7 is acidic. A pH higher than 7 is alkaline or basic.
What things are neutral?
The neutral substances that are the most well known are: water, table salt, sugar solution and cooking oil. Cooking oil is a neutral substance.
What soaps are pH neutral?
The Best pH Neutral Soap: Our PicksDove Sensitive Micellar Bar, 2 x 100 g. ... Natural Genuine Traditional hand made Aleppo Soap - Olive & Laurel Oil 200gm. ... Hygiene VOS 5 Liter Liquid Hand Soap, pH Neutral for Daily Use. ... Today Hand Soap - PH Neutral - Alkaline Free - 500ml Bottle - Pack of 6.More items...•Aug 10, 2021
What liquids are neutral?
Water and blood are both neutral. Many household cleaners, such as bleach and ammonia, are basic, as is sodium hydroxide. Citric juices, coffee and wine are acidic. Solutions with the word "acid" in them, such as stomach acid and hydrochloric acid are acidic.Mar 13, 2018
What are some examples of neutral substances?
Some of these substances are: pure water, human saliva, sodium chloride, breast milk and ammonium acetate. PH is a measure of the acidity or alkalinity of a solution.
Is breast milk water based?
More than 85% of the composition of breast milk is water based, followed by a significant presence of proteins, minerals, vitamins, fat and lactose. The pH of breast milk is neutral.
What does pH mean in chemistry?
PH is a measure of the acidity or alkalinity of a solution. If the pH is less than 7, the solution is acidic. If the pH is greater than 7, then the solution is alkaline. In the case of substances with neutral pH, this measurement is exactly equal to 7 or very close to this value.
Is sodium chloride a neutral salt?
8- Sodium Chloride (Common Salt) Table salt or common salt comes from the mixture of a strong base (NaOH) and a strong acid (HCl). When making a solution between both, the ionic balance is maintained, reason why the common chloride of sodium is considered a neutral salt.
What is the pH of pure water?
1- Pure water. In pure water the charge of hydrogen positive ions and hydroxyl negative ions is balanced. In that sense, the pH value is exactly equal to 7.
Is a sulfate a neutral salt?
It is considered as a neutral salt, as it is obtained by mixing a weak acid (acetic acid) and a weak base (ammonia). It is used in chemical analysis, in the pharmaceutical industry and as a food conservator.
What is potassium nitrate used for?
Potassium nitrate is usually used in aqueous solutions as a crop fertilizer. Assuming a composition of 13% of nitrogen and 44 or 46% of potassium oxide used in a 10% solution, a solution with a neutral pH is obtained.
What is the meaning of pH?
pH Definition. pH is defined as the negative logarithm of H + ion concentration. Hence the meaning of the name pH is justified as the power of hydrogen. We know that all the acids and bases do not react with the same chemical compound at the same rate. Some react very vigorously, some moderately while others show no reaction.
Is 7.0 a neutral pH?
As seen below, the pH scale ranges from 0 to 14 when 7.0 is neutral. It is said that water with a low pH is acidic and that water with a high pH is basic, or alkaline. Pure water should have a pH of 7.0 however, due to pollutants in the water, water supplies and precipitation appear to be slightly acidic.
What does pH mean in math?
It states that the pH equals the negative logarithmic value of the concentration of hydrogen ion (H+)
Is pH a physical parameter?
This means that the pH of water is not a physical parameter that can be measured either as a fixed, or in a quantity. Rather, it’s a nearby number of 0 and 14 that characterizes how acidic or basic a body of water is in a logarithmic scale.
What is the pH of a water source?
pH = -log [H+] A water source ‘s pH value is a function of its acidity, or alkalinity. The pH level is a function of the hydrogen atom activity, as the hydrogen activity is a reasonable indicator of the water’s acidity or alkalinity. As seen below, the pH scale ranges from 0 to 14 when 7.0 is neutral.
Does limestone neutralize acid?
The pH of a source of water can naturally vary. Some types of rock and soil, such as limestone, can more effectively neutralize acid than other rock and soil types, such as granite. Or, when large numbers of plants grow in a lake or river, when they die and decompose, they release carbon dioxide.
What is a solution with a pH of 0 to 7?
Solutions having a value of pH ranging 0 to 7 on pH scale are termed as acidic and for the value of pH ranging 7 to 14 on pH scale are known as basic solutions.
