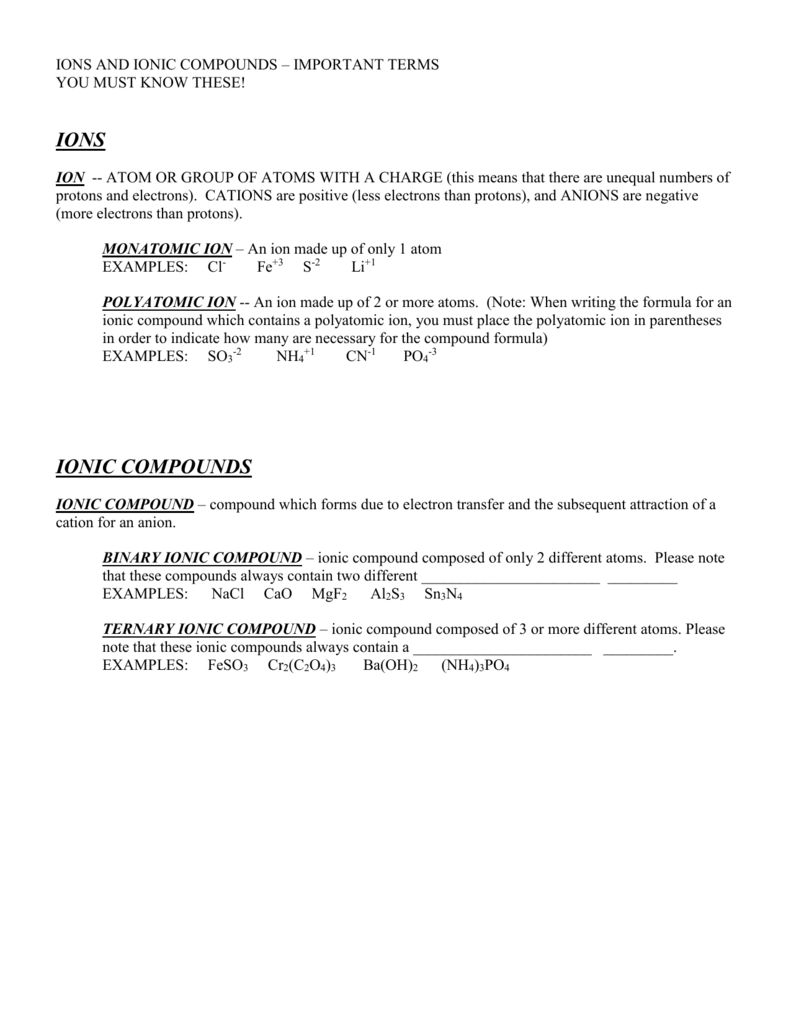
Terms
- cationA positively charged ion, as opposed to an anion.
- ionAn atom or group of atoms bearing an electrical charge, such as the sodium and chlorine atoms in a salt solution.
- anionA negatively charged ion, as opposed to a cation.
What are the different ways to form an ion?
- The phospholipid bilayer prevents ion movement into or out of the cell
- Ion channels allow ion movement across the membrane
- Electrochemical gradients drive the direction of ion flow
- At equilibrium, there is no net ion movement (but ions are still moving)
What is an ion and how is it formed?
Practice Problems
- Magnesium loses two electrons. What is its symbol?
- Oxygen gains two electrons. What is its symbol?
- Sodium loses one electron. What is its symbol?
- Carbonate is a polyatomic ion with a -2 charge. It has one carbon atom and three oxygen atoms. What is its formula?
What's the purpose of an ion?
Ions are ubiquitous in nature and are responsible for diverse phenomena from the luminescence of the Sun to the existence of the Earth's ionosphere. Atoms in their ionic state may have a different colour from neutral atoms, and thus light absorption by metal ions gives the colour of gemstones.
How to form an ion?
- Basic validation using the built in Angular Validators
- Custom validation using our own custom validators
- Multiple validations for a single input field
- Asynchronous validation (e.g. checking if a username is already taken)
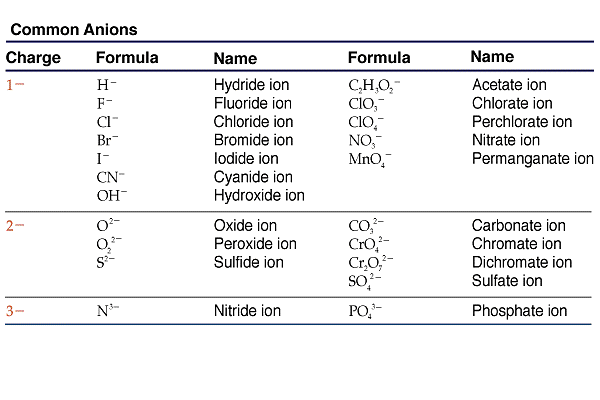
What is ion and example?
An ion is an atom or group of atoms where the number of electrons is not equal to the number of protons. That means when a stable atom gains or loses an electron, it becomes an ion. Examples of ions are as follows: H+,Na+,Ca2+,F−,O2−
Which is a ion?
0:046:51What's an Ion? - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo what are they well an ion is an atom or a group of atoms that have an electrical charge okayMoreSo what are they well an ion is an atom or a group of atoms that have an electrical charge okay that's all it is now how does this happen.
What's an ion in chemistry?
An ion is an atom or group of atoms in which the number of electron s is different from the number of proton s. If the number of electrons is less than the number of protons, the particle is a positive ion, also called a cation.
What is ion and anion?
Cations are positively-charged ions (atoms or groups of atoms that have more protons than electrons due to having lost one or more electrons). Anions are negatively-charged ions (meaning they have more electrons than protons due to having gained one or more electrons).
What are 5 examples of ions?
Many normal substances exist in the body as ions. Common examples include sodium, potassium, calcium, chloride, and bicarbonate. These substances are known as electrolytes. Ions can be created using radiation such as x-rays.
What are the 4 most common ions?
Answer and Explanation: The fours most abundant ions in the body are potassium, sodium, calcium, and chloride.
What are the types of ions?
There are two types of ions: Cations and Anions. The positively charged ions are known as cations and the negatively charged ions are known as anions.
Is h20 an ion?
H2O is not an ionic compound because the bond formed between hydrogen and oxygen is due to sharing of electrons. In ionic compounds, the bond is formed between two atoms by the exchange of electrons from one atom to another. There is no sharing of electrons involved in ionic compounds.
Q1. List Some Examples of Notation of Ions?
Ans. Let us discuss a few of the ion notation as follows:Oxide AnionThe chemical symbol of oxygen is given as O. The charge carries by the oxide an...
Q2. List Some Examples of Ions?
Ans. Examples of Polyatomic Cations - Molecular CationsA few common examples of polyatomic cations are listed below.Hydronium cation, which is deno...
Q3. List Some Examples of Anions Formed by the Organic Compounds?
Ans. A few anions can be formed through the deprotonation of some organic acids. The other anions are much known to be derived from certain organic...
Q4. List a Few Common Ions?
Ans. Some of the important and common ions can be listed as follows.Beryllium - Be₂⁺,Hydrogen - H⁺,Chromium(III), Cr₃⁺,Calcium, Ca₂⁺.
What is lithium battery?
Lithium- ion batteries, long heralded as a power grid savior capable of storing solar power for the evening, proved their worth. — Los Angeles Times, 12 July 2021 The project will also include a 70-megawatt energy storage facility powered by lithium- ion batteries.
What does ion mean in English?
English Language Learners Definition of -ion (Entry 2 of 2) : act or process. : result of an act or process. : state or condition. See the full definition for -ion in the English Language Learners Dictionary.
What is an ion?
1 : an atom or group of atoms that carries a positive or negative electric charge as a result of having lost or gained one or more electrons — see anion, cation. 2 : a charged subatomic particle (as a free electron)
What is the meaning of the term "free electron"?
2 : a charged subatomic particle (such as a free electron)
Where does the power for an EV come from?
Recent Examples on the Web: Noun The power for an EV comes from an array of lithium-ion batteries, not lead-acid batteries like the one that cranks your engine. — Motormouth Bob Weber, Star Tribune, 16 July 2021 The three Chinese companies all buy lithium-ion batteries from CATL, which is based in the southeastern province of Fujian.
How do ionizations occur?
Ionization happens by giving atoms high energy. This is done using electrical voltage or by high-energy ionizing radiation or high temperature. A simple ion is formed from a single atom. Polyatomic ions are formed from a number of atoms. Polyatomic ions usually consist of all non-metal atoms.
What are the two parts of an ionized atom?
An atom that is ionized makes two parts, one positive, and one negatively charged. For example, a neutral hydrogen atom has one proton and one electron. Ionizing the atom breaks it into two parts: (1) a positively charged hydrogen ion, H+ (2) a negatively charged electron. A liquid with ions is called an electrolyte.
Why is an ion charged?
It is " charged " so it will move near electricity. This is because atoms are made of three smaller parts: equal number of negatively-charged electrons.
What is the charge of an ion?
An ion has unequal numbers of protons and electrons. Making an ion from an atom or molecule is called ionization . The charge on a proton is chosen as +1 (positively charged). The charge on an electron is opposite to the charge on the proton. The charge on the electron is -1 (negatively charged).
What are transition metals attracted to?
They are attracted to anodes (positively charged electrodes). All simple non-metal ions (except H +, which is a proton) are anions. Transition metals can form more than one simple cation with different charges. Most ions have a charge of less than 4, but some can have higher charges.
What does an ion mean in Greek?
In Greek ion is like the word "go". "Anion" and "cation" mean "up-goer" and "down-goer". "Anode" and "cathode" are "way up" and "way down".
Do metal ions move?
For example, in a wire, the metal ions do not move, but the electrons move as electricity. A positive ion and a negative ion will move together. Two ions of the same charge will move apart. When ions move they also make magnetic fields . Many ions are colourless.
What is the driving force behind ionic bond formation?
It is important to make a note that the electrostatic forces of attraction which arise between the positively charged cations and negatively charged anions are described as the driving force behind the ionic bond formation. When the 2 oppositely charged ions produce an ionic bond with each other, the resulting compound type is commonly known as an ionic compound.
What is the name of an ion that holds a greater electron count than protons?
On the other side, ions that hold a greater electron count than protons are known to contain a net negative charge. Commonly, these ions are referred to as anions .
How do ions form?
The other important process via which ions can be formed is done by using chemical interactions. As an example, when an ionic compound like salt is dissolved in a suitable solvent (like water), the atoms that constitute that salt undergoes dissociation and produce free ions. However, when common salt, which is also called sodium chloride, is dissolved in water, it undergoes dissociation to generate chloride anions and sodium cations. It should also be noted that the symbol Cl denotes the chloride anions– and sodium cations are denoted using the symbol Na+.
What is the symbol for lithium cation?
Lithium Cation. The chemical symbol of the lithium atom is given as Li. Because the lithium cation carries a net charge of +1, the monoatomic ion is represented as Li⁺. Q2.
What does the symbol Cl mean in chemistry?
It should also be noted that the symbol Cl denotes the chloride anions– and sodium cations are denoted using the symbol Na+. Another important notable process via which ions can be formed is the passage of direct currents via some conducting solutions, which results in the production of ions in the solution.
What is an ion?
An ion can be described as a chemical species that hold either a positive or negative charge of some amount of magnitude. The word 'ion' can be used to refer to molecules or atoms that hold non-zero net charges associated with them. Thus, all the ions contain either a greater number of protons compared to electrons in their overall molecular ...
What are some examples of anions derived from organic compounds?
Some common examples of anions derived from organic compounds can be listed as follows. Acetate anion, which is denoted using the chemical formula CH₃COO⁻. Formate anion, which is denoted using the chemical formula HCOO⁻. Q4.
What is the purpose of lithium salts?
Lithium salts provide the ion s that move through the new electrolyte.
What is an ion in chemistry?
noun Physics, Chemistry. an electrically charged atom or group of atoms formed by the loss or gain of one or more electrons, as a cation (positive ion ), which is created by electron loss and is attracted to the cathode in electrolysis, or as an anion (negative ion ), which is created by an electron gain and is attracted to the anode.
What is the valence of an ion?
The valence of an ion is equal to the number of electrons lost or gained and is indicated by a plus sign for cations and a minus sign for anions, thus: Na +, Cl−, Ca ++, S =. one of the electrically charged particles formed in a gas by electric discharge or the like.
What is the charge of salt?
Each molecule of salt contains a pair of ion s, atoms with an electrical charge .
What is the difference between positive and negative ions?
An atom or a group of atoms that has an electric charge. Positive ions, or cations, are formed by the loss of electrons; negative ions, or anions, are formed by the gain of electrons.
What does "suffix" mean in Latin?
a suffix, appearing in words of Latin origin, denoting action or condition, used in Latin and in English to form nouns from stems of Latin adjectives (communion; union), verbs (legion; opinion), and especially past participles (allusion; creation; fusion; notion; torsion).
What is the ancestor of the Ionians?
noun. Classical Mythology. the eponymous ancestor of the Ionians: a son of Apollo and Creusa who is abandoned by his mother but returns to become an attendant in Apollo's temple at Delphi.
What are positive and negative ions called?
Positive and negative ions have special names. Positive ions are called cations and negative ions are called anions . A way to remember which is which is to think of the prefix "anti", which generally has a negative connotation, just like an anion has a negative charge.
How many oxygen atoms are in carbonate?
Carbonate is a polyatomic ion with a -2 charge. It has one carbon atom and three oxygen atoms. What is its formula?
How many atoms are in a monoatomic ion?
Monoatomic ions are made up of one atom with a charge ( {eq}H^+ , Cl^- {/eq}).
Why do nonmetals gain electrons?
Nonmetals most often gain electrons to stabilize, becoming anions. It would require less energy to gain electrons to be like the noble gas in their period as opposed to losing electrons to have an electron structure of the previous noble gas like the metals do. For example, fluorine is one electron away from having an electron structure like neon, but 7 away from being like the previous gas, helium.
Why is an ion positively charged?
An anion has more electrons and is negatively charged; a cation is positively charge because it has lost electrons.
What is a molecule?
Molecules are any two or more atoms that are bonded together. The atoms in a molecule can be the same, like in hydrogen gas (H 2 ), or different, like in water (H 2 O). Compounds must be made up of at least two different atoms that are bonded together. Both compounds and molecules can carry a positive or negative charge. When they do, they are also considered ions. The charge of an atom or molecule affects how it will behave in the presence of other particles and how it will bond.
What are the parts of an atom?
There are three parts to the atom. The nucleus of the atom has protons and neutrons, while the electron orbits a space around the nucleus. The positively charged particle as a proton. The negatively charged particle is an electron. The neutron is neutral, as its name suggests.
What is an ion in science?
In science, an ion is an atom or molecule with an electric charge. Outside of the Chemistry classroom, though, ion is a black slang rendering of I don't. As in ion wanna take another Chemistry class.
What does the ion mean in Greek?
Memedroid. Perhaps there are some of you who read the above paragraph and said Ion know what that means. This ion means “I don’t.”. It is a spelling based on the colloquial pronunciation of I don’t, especially in Black English.
Why are chemical ions important?
The chemical ion helps make the world go round, even if baffles many a student in high school . They are key to understanding chemical bonds.
What does "ion" mean in slang?
ION sees occasional use among the many abbreviations of internet slang, from LOL to STFU. Typically standing for “in other news, ” ION signals a change of topic, often abrupt in nature and with a degree of self-deprecation.
When were ions discovered?
TeePublic. The great British scientist Michael Faraday discovered ions in 1834. By the loss or gain of electrons, atomic or molecular ions carry a positive or negative charge, which attracts them to oppositely charge particles to form various chemicals.
Is "ion" a formal word?
This is not meant to be a formal definition of ion like most terms we define on Dictionary.com, but is rather an informal word summary that hopefully touches upon the key aspects of the meaning and usage of ion that will help our users expand their word mastery.
Why is an ion charged?
An ion is a charged atom or molecule. It is charged because the number of electrons do not equal the number of protons in the atom or molecule. An atom can acquire a positive charge or a negative charge depending on whether the number of electrons in an atom is greater or less then the number of protons in the atom.
What is it called when an atom is attracted to another atom?
When an atom is attracted to another atom because it has an unequal number of electrons and protons, the atom is called an ION. If the atom has more electrons than protons, it is a negative ion, or ANION. If it has more protons than electrons,it is a positive ion. What is an atom?
