Full Answer
What are some examples of ionic bonds?
Some ionic bond examples include:
- NaCl: sodium chloride
- NaBr: sodium bromide
- NaF: sodium fluoride
- NaI: sodium iodide
- KF: potassium fluoride
- KCl: potassium chloride
- KI: potassium iodide
- KBr: potassium bromide
- LiI: lithium iodide
- Li2O: lithium oxide
What are 5 examples of ionic compounds?
Ionic bond examples include:
- LiF - Lithium Fluoride
- LiCl - Lithium Chloride
- LiBr - Lithium Bromide
- LiI - Lithium Iodide
- NaF - Sodium Fluoride
- NaCl - Sodium Chloride
- NaBr - Sodium Bromide
- NaI - Sodium Iodide
- KF - Potassium Fluoride
- KCl - Potassium Chloride
What are the characteristics of ionic bonds?
- The ionic bonds are the strongest of all the bonds.
- The ionic bond has charge separation, and so they are the most reactive of all the bonds in the proper medium.
- The ionic bonded molecules have high melting and boiling point.
- The ionic bonded molecules in their aqueous solutions or in the molten state are good conductors of electricity. ...
How do atoms form an ionic bond?
What is Ionic Bond?
- Forming an Ionic Bond. The chemical bond that is formed between 2 atoms through the transfer of one or more electrons from the electropositive or metallic element to the atom ...
- Writing Formula of an Ionic Compound. The cation and the anion should follow the octet rule for maximum stability. ...
- Conditions for Formation of Ionic Bond. ...

What is an ionic bond? Explain with an example?
When a positively charged ion forms a bond with a negatively charged ion, one atom donates electrons to the other, this is known as an ionic bond....
What kind of force is present in ionic bonds?
Ionic bonding is a form of chemical connection in which one atom loses valence electrons and gains them from another. For both atoms involved, this...
How do you find ionic formulas?
To obtain an ionic compound’s formula, first determine the cation and record its symbol and charge. Then, write down the anion’s symbol and charge...
Are ionic bonds between two nonmetals?
Consider whether each element is a metal or a nonmetal to anticipate the sort of bond that will form between them. In general, nonmetals form coval...
How do you break ionic bonds?
Ionic chemicals dissolve in polar solvents like water because they are polar. When polar solvents disrupt the ionic bonds, they dissolve. By dissol...
What is a covalent bond?
A covalent bond is formed by the sharing of electrons between participating atoms. In it, each atom contributes an equal number of electrons for bo...
Can an ionic bond form between two metals?
No, the ionic bond can not form between two metals. An ionic bond is formed between positive and negative ions or a metal and a non-metal by the do...
How are ionic bonds formed between atoms?
Ionic bonds are formed by the complete transfer of electrons between two atoms. An atom that gains an electron acquires a negative charge and is kn...
Do ionic bonds have a high melting point?
Yes, ionic bonds have a high melting point. Ionic bonds are formed by the complete transfer of electrons held together by a strong electrostatic fo...
What is an ionic bond explained with an example?
When the electronegativity difference between the two atoms is large, they can attain octets by the complete transfer of one or more electrons from...
What are 3 examples of an ionic bond?
The three examples of ionic bonds are Lithium Fluoride, Sodium Chloride, and Lithium Bromide.
Are ionic bonds strong?
Ionic bonds are stronger than covalent bonds due to the coulombic attraction between ions of opposite charges.
How do you explain ionic bonding?
Ionic bonding is the complete transfer of valence electron(s) between atoms. It is a type of chemical bond that generates two oppositely charged ions.
What are the 4 properties of ionic compounds?
They form crystals. They have high melting points and high boiling points. They have higher enthalpies of fusion and vaporization than molecular co...
What is the ionic bond in sodium chloride?
ionic bond: sodium chloride, or table salt. Ionic bonding in sodium chloride. An atom of sodium (Na) donates one of its electrons to an atom of chlorine (Cl) in a chemical reaction, and the resulting positive ion (Na +) and negative ion (Cl −) form a stable ionic compound ...
How many electrons does sodium have?
The sodium atom has a single electron in its outermost shell, while chlorine needs one electron... Ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals and the alkali and alkaline-earth metals.
What is an ionic bond?
See Article History. Alternative Titles: electrovalency, electrovalent bond, heteropolar bond, polar bond. Ionic bond, also called electro valent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence ...
Which atom has a single electron in its outermost shell?
Sodium chloride exhibits ionic bond ing. The sodium atom has a single electron in its outermost shell, while chlorine needs...
When do ionic and covalent bonds form?
Ionic bonds typically form when the difference in the electronegativities of the two atoms is great, while covalent bonds form when the electronegativities are similar. Compare covalent bond. The Editors of Encyclopaedia Britannica This article was most recently revised and updated by Adam Augustyn, Managing Editor, Reference Content.
What is an encyclopedia editor?
Encyclopaedia Britannica's editors oversee subject areas in which they have extensive knowledge, whether from years of experience gained by working on that content or via study for an advanced degree. ...
Is an ionic crystal hard or nonvolatile?
The magnitude of the electrostatic forces in ionic crystals is considerable. Accordingly, these substances tend to be hard and nonvolatile.
What is an Ionic Bond?from byjus.com
The electrostatic force of attraction which holds the two oppositely charged ions together is called the ionic bond.
What happens to metals in a reaction?from byjus.com
In a reaction between metals and non-metals, metals generally loose electrons to complete their octet while non-metals gain electrons to complete their octet. Metals and non-metals generally react to form ionic compounds.
How are ionic solids held together?from byjus.com
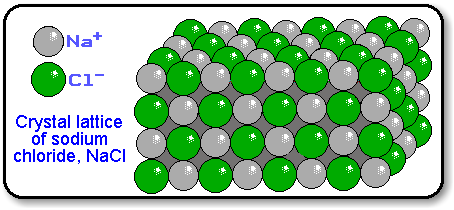
Ionic solids are held together by the electrostatic attraction between the positive and negative ions. For example, the sodium ions attract chloride ions and the chloride ion attracts sodium ions. The result is a three-dimensional structure of alternate Na + and Cl – ions. This is a crystal of sodium chloride.
What type of bond is formed when the valence (outermost) electrons of one atom are transferred?from britannica.com
Ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom.
Why is the crystal of sodium chloride uncharged?from byjus.com
This is a crystal of sodium chloride. The crystal is uncharged because the number of sodium ions is equal to the number of chloride ions. The forces of attraction between the ions hold them in the structures. These ionic bonds between the charged particles result in a giant structure of ions.
What type of bond does each element have?from byjus.com
Consider whether each element is a metal or a nonmetal to anticipate the sort of bond that will form between them. In general, nonmetals form covalent bonds, metals and nonmetals create ionic connections, and metals and metals create metallic connections.
What is the term for the transfer of electrons between atoms?from byjus.com
Electrovalent Bond . Electrovalent bonds are produced when electrons are transferred from atoms of one element to atoms of another element, producing positive and negative ions. The bond which is formed by the transfer of electrons between the atoms is called electrovalent bond or ionic bond.
Ionic Bonding Diagram
From this diagram, one can easily understand that an ionic molecule is formed when a metal atom transfers its electrons to a non-metal atom.
Electrovalent Bond
While transferring electrons from one atom to another, bonds are formed. These bonds are referred to as electrovalent bonds or ionic bonds. They will contain positive and negative ions. Usually, electrovalent bonds are formed only between non-metals and metals. Also, it is impossible to form a bond between two non-metals.
Ionic Bonding and Electronegativity
An ionic or electrovalent bond is formed while transferring valence electrons to acquire stability completely.
Electronegativity
It is nothing but the tendency of an atom to attract the electron shared pairs towards itself is called electronegativity. Electronegativity is a dimensionless property because it does not involve any dimension and is only a capability.
What is Ionic Bond?
When the electronegativity difference between the two atoms is large, they can attain octets by the complete transfer of one or more electrons from one atom to the other and can be linked together by an attractive force known as an ionic bond or electrovalent bond.
What is the tendency of atoms of various elements to attain a stable electronic configuration of eight electrons in their?
The tendency or urge of atoms of various elements to attain a stable electronic configuration of eight electrons in their valence shells is the cause of chemical combinations .
What is the number of electrons lost or gained by an atom called?
In the formation of an electrovalent bond, the number of electrons lost or gained by an atom is called electrovalence or electrovalency.
How many electrons does helium have?
All noble gases except helium have 8 electrons in their valence shell, i.e., the valence shell is completely filled and hence has no tendency to gain or lose electrons. The valency of noble gases is zero. Completely filled valence shell corresponds to maximum stability. All other elements except noble gases have incompletely filled valence shells and hence combine with other elements to attain a stable 8 electron configuration. It is to be noted that helium is also a noble gas and its stable electronic configuration has only 2 electrons in the valence shell.
What happens to electrons in an ionic bond?
In an ionic bond, electrons are either lost or gained to attain noble gas electronic configuration.
Why do atoms combine?
According to the modern view, the atoms combine to form chemical bonds to acquire a state of minimum energy. It is the natural tendency of every object in this universe to gain minimum energy and maximum stability. Hence, atoms combine with each other to lower their energy.
How many kinds of chemical bonds are there?
As per the classification, majorly there are 2 kinds of chemical bonds.
What is the electronegativity of sodium chloride?
Electronegativity. The difference in electronegativity between the metal and non metal should be high enough to form an ionic compound. For example, the electronegativity of Sodium and chlorine in sodium chloride is: Na = 0.9 and Cl = 3.0.
How is sodium chloride formed?
An electrostatic force holds these to atoms to together in a crystallographic lattice. Thus sodium chloride is formed. Another example of ionic bond is the formation of magnesium chloride by transferring two electrons from a magnesium atom to two chlorine atoms. Mg + Cl 2 → Mg 2+ + 2Cl – → MgCl 2.
What is an ionic bond?
Ionic bond is a kind of chemical bond which involves an electrostatic attraction between two oppositely charged ions because of the complete transfer of valence electrons between them. As for example: metals such as sodium losses electrons to to become positive ion, whereas non-metal such as chlorine accepts electrons ...
Why do metals form ionic bonds?
Ionic bond occurs between metals and non-metals because the metals have only few electrons in its outermost shell. Thus it likes to give away these electron to achieve the noble gas configuration and satisfy the octet rule. The number of electrons , a metal loses is the number of positive charge it achieves to form the ionic bond .
How to form an ionic bond?
So that the atom will have more tendency to accept the electron to release more energy to the nature and to become more stable ionic compound. B + e – → B + EA.
What is the energy required to remove a valence electron from the outermost shell of a metal atom?
The ionization energy is the energy required to remove a valence electron from the outermost shell of a metal atom. This required energy should be low to form an ionic bond.
Which metal gives electrons?
As for example: metals such as sodium losses electrons to to become positive ion, whereas non-metal such as chlorine accepts electrons to become a negative ion. The metal that gives electrons is called donor and the non-metal that accepts electrons is called acceptor.
What is an Ionic Bond?from byjus.com
The electrostatic force of attraction which holds the two oppositely charged ions together is called the ionic bond.
What happens when ions approach each other?from embibe.com
When these ions approach each other, due to the increased nuclear charge on the cation, it attracts the loosely bound outermost electrons of the large anion. As a result, the electron cloud of the anion gets distorted by its neighbouring cation. This process is known as Polarisation, and the ability of the cation to attract the electron cloud of the anion is called its polarising power. The tendency of the anion to get polarised by the cation is called polarizability.
What happens when sodium chloride is added to water?from embibe.com
Solubility in water – When an ionic compound like sodium chloride is added to water, the negative end of the water molecule attracts the cations and pulls them out of the crystal. Likewise, The positive end of the water molecule pulls the anions resulting in the dissolution of the compound in water.
What is the ionic bond of table salt?from embibe.com
We are all quite familiar with table salt that we use to add flavour to food. We also know that chemically, table salt is sodium chloride in which sodium and chloride ions are bonded together by ionic bonds. Various factors affect the formation of these ions and consequently ionic bonding, which ultimately gives rise to ionic compounds. The factors are –
What type of bond is formed when one atom gains electrons while the other atom loses electrons from its?from byjus.com
The bond formed by this kind of combination is known as an ionic bond or electrovalent bond. This kind of bond is formed when one atom gains electrons while the other atom loses electrons from its outermost level or orbit.
How many electrons does sodium have?from britannica.com
The sodium atom has a single electron in its outermost shell, while chlorine needs one electron... Ionic bonding results in compounds known as ionic, or electrovalent, compounds, which are best exemplified by the compounds formed between nonmetals and the alkali and alkaline-earth metals.
What type of bond is formed when the valence (outermost) electrons of one atom are transferred?from britannica.com
Ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom.
The Formation of Ionic Compounds
Binary ionic compounds are composed of just two elements: a metal (which forms the cations) and a nonmetal (which forms the anions). For example, NaCl NaCl is a binary ionic compound. We can think about the formation of such compounds in terms of the periodic properties of the elements.
Key Concepts and Summary
Atoms gain or lose electrons to form ions with particularly stable electron configurations. The charges of cations formed by the representative metals may be determined readily because, with few exceptions, the electronic structures of these ions have either a noble gas configuration or a completely filled electron shell.
Try It
Does a cation gain protons to form a positive charge or does it lose electrons?
What is a ionic bond in simple terms?
ionic bond, also called electrovalent bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom.
What is an ionic bond example?
An example of an ionic bond is the bond in sodium chloride, which is salt. Sodium’s valence electron is transferred to the outer electron shell of chloride. Molecules with ionic bonds form ionic compounds.
What are the 3 types of bonds?
There are three primary types of bonding: ionic, covalent, and metallic.
What is meant by covalent bond?
covalent bond, in chemistry, the interatomic linkage that results from the sharing of an electron pair between two atoms. The binding arises from the electrostatic attraction of their nuclei for the same electrons.
