
When should Aseptic Process simulation be performed?
Bioreactors And Fermenters This type of aseptic process simulation is usually only performed during commissioning of a new bioreactor or fermenter, or if a major change is made to a bioreac - 42 Journal ofValidationtechnology[Winter2010]ivthome.com
What is aseptic processing?
Aseptic processing. Aseptic processing is the process by which a sterile ( aseptic) product (typically food or pharmaceutical) is packaged in a sterile container in a way that maintains sterility.
How can pharmalex help with aseptic process simulations?
The PharmaLex team can also provide you with support and guidance on the establishment, implementation or improvement of your Aseptic Process Simulations. If you would like further information or wish to discuss how we can tailor our services to assist you, please connect with us to discuss +353 1 846 4742 or [email protected]
How should the aseptic compounding process be simulated?
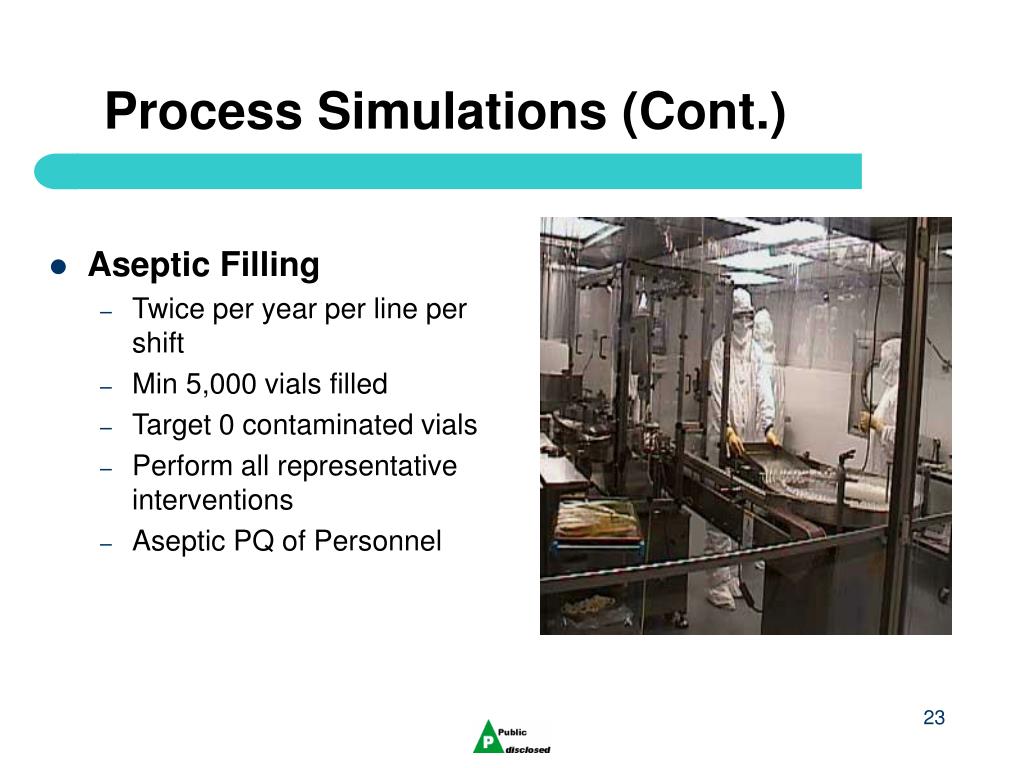
The entire aseptic compounding process should be simulated including all aseptic additions. APS can be stand alone for the compounding step or integrated with filling. Filling
What is aseptic process in pharma?
Aseptic processing is defined as “handling of sterile product, containers, and/or devices in a controlled environment, in which the air supply, materials, equipment, and personnel are regulated to maintain sterility” (ISO 13408-1, 2008).
What is aseptic media fill?
A media fill is the performance of an aseptic manufacturing procedure using a sterile microbiological growth medium, in place of the drug solution, to test whether the aseptic procedures are adequate to prevent contamination during actual drug production.
What is media fill PPT?
What is Media Fill ? The Media fill or Broth fill technique is one in which a liquid microbiological nutrient growth medium is prepared and filled in a simulation of normal manufacturing operation.
How do you investigate media fill failure?
Review the history of media fill for the product last time for contamination. Review the batch filled with the same filling line since last media fill test. List all possible root causes of failure. List the batches to be held, those are suspicious and require re-analysis.
What is aseptic process validation?
aseptic process validation. risk assessment. Aseptic process simulations, sometimes referred to as media fills, are studies conducted on the aseptic filling process. The process is simulated or run as closely to the actual production procedure as possible. Product is replaced with growth media.
What is cleaning validation in pharma?
Cleaning validation is a procedure of establishing evidence that cleaning processes for manufacturing equipment prevents product contamination. Cleaning validation should be properly documented to demonstrate Current Good Manufacturing Practice (CGMP) for finished pharmaceuticals.
Why compressed air is used in media fill?
As an example, if your product manufacturing employs Nitrogen or Argon purging, for media fills you must choose to purge the broth with compressed air. This way you ensure that contamination if any due to wrong aseptic practices / interventions etc.
What is aseptic area?
An aseptic area is a premise in a clean area, designed, constructed, serviced and used with an intention to prevent the microbial contamination of the product.
Why nitrogen is not used in media fill?
Use of nitrogen can affect the growth of microorganisms or better to say growth promotion properties in media. Hence you can get a false negative growth in the filled unit. So, it is not accepted doing vacuum break through nitrogen in lyophilizer .
How do you investigate sterility test failures?
1 are as follows:Identification (speciation) of the organism isolated from the sterility test (a strain level ID is desirable for such investigations)Record of laboratory tests and deviations.Monitoring of production area environment.Personnel monitoring.Product pre-sterilization bioburden.Production record review.More items...•
How do I select media fill batch size?
For small batches, the number of containers for media fills should at least equal the size of the product batch. The target should be zero growth and the following should apply: ➢ When filling fewer than 5000 units, no contaminated units should be detected.
Is media fill required for terminally sterilized products?
❖ Media fill is not required for terminally sterilized (TS) product(s). Even when TS product shares the same filling line with other aseptic product, the media fill for TS product is not required.