
An atom is made of three parts – protons, neutrons and electrons. Each of these parts has an associated charge. The protons carry a positive charge, electrons have a negative charge and neutron possess no charge. Protons and neutrons make up the nucleus of the atom and electrons orbit the nucleus at different energy levels.
What is an atom in science for kids?
Science >> Chemistry for Kids. The atom is the basic building block for all matter in the universe. Atoms are extremely small and are made up of a few even smaller particles. The basic particles that make up an atom are electrons, protons, and neutrons.
What is meant by atomic structure?
Atomic structure refers to the structure of atom comprising of a nucleus (center) in which the protons (positively charged) and neutrons (neutral) are present.
What is the atomic structure of matter made of?
Primarily, the atomic structure of matter is made up of protons, electrons and neutrons. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom.
What is the atomic number of an atom?
The atomic number is the total of protons that live in the center of an atom. It is principal property. It shows how many protons the atom has in its center. This characteristic defines the kind of an atom. In the periodic table, this number appears above the element symbol.
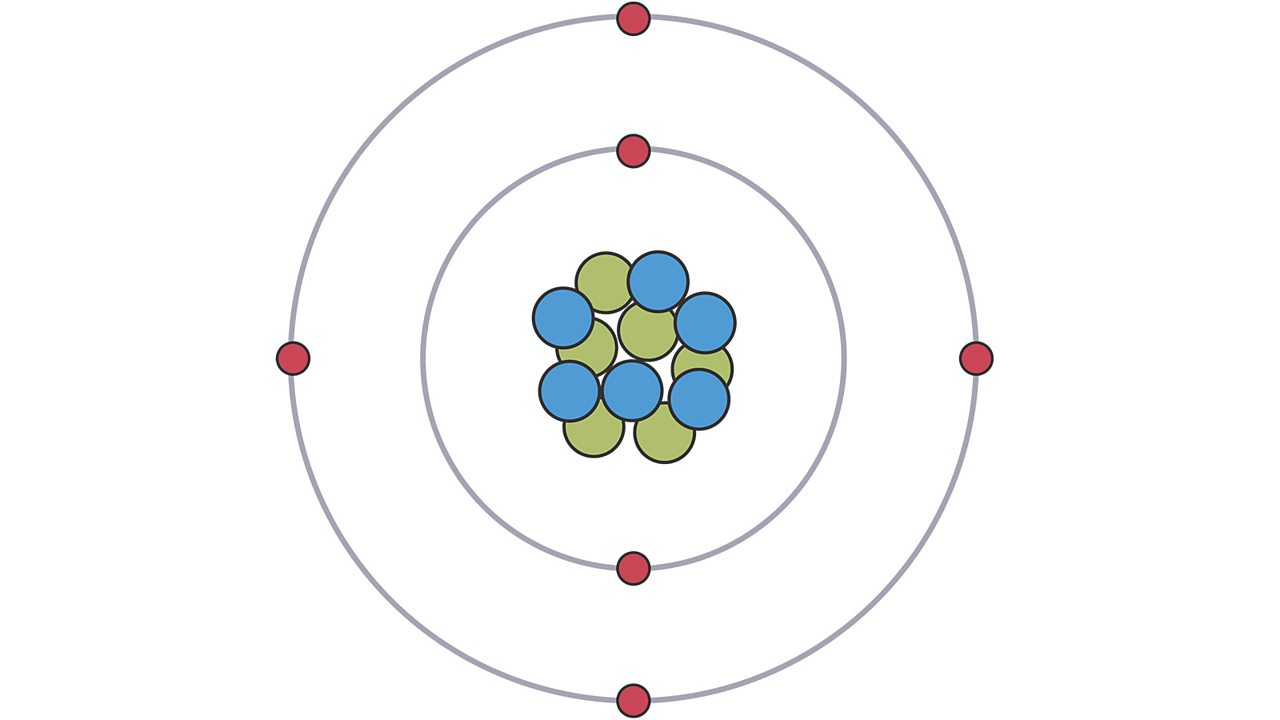
What is atomic structure in simple words?
Atomic structure refers to the structure of an atom comprising a nucleus (centre) in which the protons (positively charged) and neutrons (neutral) are present. The negatively charged particles called electrons revolve around the centre of the nucleus.
What does atomic mean for kids?
The smallest unit of an element. Consists of a central nucleus surrounded by one or more electrons. Matter is made up of atoms. An atom is the smallest whole particle of matter. Subatomic particles are smaller than atoms and are the tiny particles that an atom is made of.
What is atomic structure and periodic table?
Atomic structure is the make-up of an atom and what it consists of. An atom is a central positively charged nucleus that is made of protons and neutrons. Around this nucleus is a number of electrons that differs depending on the element of the periodic table.
What is the best definition of atomic?
Definition of atomic of, pertaining to, resulting from, or using atoms, atomic energy, or atomic bombs: an atomic explosion. propelled or driven by atomic energy: an atomic submarine. Chemistry. existing as free, uncombined atoms.
How do you define atomic?
Atomic means relating to power that is produced from the energy released by splitting atoms. ... atomic energy. Atomic means relating to the atoms of substances.
Why is atomic structure important?
By learning about atomic structure, we can find out how atoms combine and form many compunds. By learning about atomic structure, we can find out how atoms collide. By learning about atomic structure, we can find out why atoms do not have mass.
Who discovered atomic structure?
The modern atomic theory, which has undergone continuous refinement, began to flourish at the beginning of the 19th century with the work of the English chemist John Dalton.
How do you draw atomic structure?
3:005:12How to Draw an Atom! - YouTubeYouTubeStart of suggested clipEnd of suggested clipBut. How you place them depends on which model the atom you're following I mean depend on theMoreBut. How you place them depends on which model the atom you're following I mean depend on the purpose of your drawing. It might be good enough to randomly plunk the electrons around the nucleus.
What is atomic in a sentence?
In logic and analytic philosophy, an atomic sentence is a type of declarative sentence which is either true or false (may also be referred to as a proposition, statement or truthbearer) and which cannot be broken down into other simpler sentences.
Why is it called atomic?
The term "atom" comes from the Greek word for indivisible, because it was once thought that atoms were the smallest things in the universe and could not be divided.
How do you explain elements to children?
A chemical made up of only one kind of atom is called an element. There are 118 different elements, although only 98 of them are found naturally on Earth. Some elements, such as gold, are found in their pure form on Earth. Others, like iron, have to be separated from other substances.
How do you use atomic in a sentence?
The atomic weight of the element has been determined by analysis. The atomic weight was determined by Cleve.
How small is an atom?
Scientists measure the size of atoms in nanometers. It varies from 0,1 to 0,5 nanometers in width. The only way to see atoms is experimental. Scientists use special tools and lead experiments to watch how atoms work.
What is the main property of atoms?
The main property of atoms is indivisibility. Imagine an apple. We can easily cut it on two halves, then on quarters. But what if we continue to cut it on the smallest parts until you can not split it any further? So, a particle that we cannot divide further is suggested to be indivisible. And this is an atom.
Why do atoms lose electrons?
However, some atoms can lose electrons or gain additional ones due to their interaction in chemical reactions or collisions with different particles. As a result, the atom transfers into a new particle with a positive or negative charge.
How did the researchers discover new particles?
The researchers used particle accelerators. During their experiments, electrons fired at the protons. As a result, they bounced off tiny particles inside protons. The discovery of new particles has taken four years of experiments.
When an atom gets rid of one or more electrons, the amount of protons becomes dominant?
When an atom gets rid of one or more electrons, the amount of protons becomes dominant, and it shifts to the positive charge. It is a cation.
How many hydrogen atoms are in a water molecule?
Two hydrogen atoms + one oxygen atom = molecule of water.
Why are quarks called fundamental particles?
Tip to note: quarks are named fundamental particles because they have no substructure and are indivisible.
What is Atomic Structure?
The atomic structure of an element refers to the constitution of its nucleus and the arrangement of the electrons around it. Primarily, the atomic structure of matter is made up of protons, electrons and neutrons.
How to tell the structure of an atom?
The structure of atom of an element can be simply represented via the total number of protons, electrons, and neutrons present in it . The atomic structures of a few elements are illustrated below.
What are the protons and neutrons in an atom?
The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. The atomic number of an element describes the total number of protons in its nucleus. Neutral atoms have equal numbers of protons and electrons. However, atoms may gain or lose electrons in order to increase their stability and ...
What happens to the energy of the electrons when they revolve around the nucleus?
If electrons have to revolve around the nucleus, they will spend energy and that too against the strong force of attraction from the nucleus, a lot of energy will be spent by the electrons and eventually, they will lose all their energy and will fall into the nucleus so the stability of atom is not explained.
What are the particles inside an atom called?
The discovery of particles inside atoms led to a better understanding of chemical species, these particles inside the atoms are called subatomic particles.
How many neutrons are in a hydrogen atom?
Structure of Hydrogen atom: This implies that it contains one proton, one electron, and no neutrons ( total number of neutrons = mass number – atomic number)
What were the atomic models?
Atomic Models. In the 18th and 19th centuries, many scientists attempted to explain the structure of the atom with the help of atomic models. Each of these models had their own merits and demerits and were pivotal to the development of the modern atomic model.
What Are Atoms?
You can't see atoms, but they make up everything around you: your chair, your desk, the birds outside, even you! Atoms are the foundation of matter, which is everything that makes up the universe around us. Each kind of atom makes up a pure substance called an element. You may have heard of oxygen, lead, and gold. Well, these are all examples of elements. Can you believe that a pure gold necklace is made up of billions of gold atoms?
What are the particles in an atom?
Structure of an Atom. If you look inside an atom, you find tiny particles called protons, neutrons, and electrons. Atoms of each element have different numbers of these particles. Protons and electrons have electrical charges.
What are the two parts of an atom that spin around the nucleus?
Protons and neutrons form the center of an atom, called the nucleus. Electrons spin around outside the nucleus in spaces called electron shells. These aren't shells like you'd find at the beach, though. That's just the name given to the different layers of space the electrons occupy.
What is the center of an atom?
Every atom has a center called a nucleus, which is made of particles called protons and neutrons. Electrons move in electron shells around the nucleus. Atoms can bond to one another to form solids, liquids, or gases. To unlock this lesson you must be a Study.com Member. Create your account.
What happens when atoms have more room to move?
If atoms have more room to move, they form liquids. You can imagine this by thinking about how much more you could move if everybody were to get out of the elevator except you and two of your friends. Atoms that are able to move freely form gases, which do not have any defined shape.
What is the smallest building block in the universe?
This lesson will introduce you to the atom, which is the smallest building block of everything in our universe. You will learn about the basic structure of atoms and how they form different substances. Updated: 01/10/2020
Do atoms have defined shapes?
Atoms that are able to move freely form gases, which do not have any defined shape. Now imagine the elevator stops at the ground floor and the doors open to a really spacious lobby with many hallways; you and your friends might not stay together because you have a lot of room to wander about. This is the same idea when gases form.
What is the nucleus of an atom?
But the nucleus is very hard to split, meaning most atoms are around for a long time. At the center of the atom is the nucleus. The nucleus is made up of the protons and neutrons. The electrons spin in orbits around the outside of the nucleus. The proton is a positively charged particle that is located at the center of the atom in the nucleus.
How many atoms are there in an element?
Each different kind of atom makes up an element. There are 92 natural elements and up to 118 when you count in man-made elements. Atoms last a long time, in most cases forever. They can change and undergo chemical reactions, sharing electrons with other atoms.
How many atoms are there in the human body?
There are so many atoms in a single human body we won't even try to write the number here. Suffice it to say that the number is trillions and trillions (and then some more). There are different kinds of atoms based on the number of electrons, protons, and neutrons each atom contains. Each different kind of atom makes up an element.
What is the charge of an atom?
If there are the same number of electrons and protons in an atom, then the atom is said to have a neutral charge . Electrons are attracted to the nucleus by the positive charge of the protons. Electrons are much smaller than neutrons and protons. About 1800 times smaller!
How many types of quarks are there?
There are 6 types of quarks: up, down, top, bottom, charm, and strange. Neutrino - Neutrinos are formed by nuclear reactions. They are like electrons without any charge and are usually travelling at the speed of light. Trillions and trillions of neutrinos are emitted by the sun every second.
What are the basic building blocks of matter?
The Atom. The atom is the basic building block for all matter in the universe. Atoms are extremely small and are made up of a few even smaller particles. The basic particles that make up an atom are electrons, protons, and neutrons.
Which atom has a single proton?
The Proton. The proton is a positively charged particle that is located at the center of the atom in the nucleus. The hydrogen atom is unique in that it only has a single proton and no neutron in its nucleus. The Electron. The electron is a negatively charged particle that spins around the outside of the nucleus.
What is the most important thing about an atom?
The single most important thing to know about an atom is how many protons it has in its nucleus. This is known as its atomic number. The atomic number determines what kind of atom it is. Every atom is associated with a specific chemical element. An atom is the smallest unit of an element, and each chemical element has a unique atomic number. For instance, hydrogen has an atomic number of 1 because every hydrogen atom has one proton in its nucleus. No other element has an atomic number of 1.
What are the subatomic particles in an atom?
These are called subatomic particles. At the center of an atom is a nucleus. The nucleus consists of protons and neutrons. Protons carry a positive electrical charge, while neutrons carry no electrical charge. Together, protons and neutrons are called nucleons.
What would happen if an atom did not have 6 protons?
If it did not have six protons, it would not be carbon. An ordinary atom has an equal number of protons and electrons. Thus the positive and negative charges are balanced. Some atoms, however, lose or gain electrons in chemical reactions or in collisions with other particles.
What are protons and neutrons made of?
Together, protons and neutrons are called nucleons. Surrounding the nucleus is a cloud of negatively charged electrons. Scientists believe that subatomic particles—protons, neutrons, and electrons—are themselves made up of smaller substances. The substances are called quarks and leptons.
How are mass number and atomic weight similar?
The mass number is equal to the total number of protons and neutrons in an atom. The atomic weight is equal to the mass number divided by a certain number that scientists have come up with. The mass number and atomic weight are very similar. For example, for carbon, the mass number is 12, and the atomic weight is 12.011.
Why does hydrogen have an atomic number of 1?
For instance, hydrogen has an atomic number of 1 because every hydrogen atom has one proton in its nucleus. No other element has an atomic number of 1. Two other related properties of atoms are the mass number and the atomic weight. The mass number is equal to the total number of protons and neutrons in an atom.
What are the building blocks of matter?
Introduction. The tiny particles called atoms are the basic building blocks of all matter. Atoms can be combined with other atoms to form molecules, but they cannot be divided into smaller parts by ordinary means. The word atom comes from the Greek word atomos, meaning “indivisible.”. The ancient Greeks were the first to think ...
