What are some everyday examples of Boyles law?
Top 6 Applications Of Boyle’s Law
- Spray Paint
- The mechanics of human breathing
- Working of syringe
- Storage of gas
- Bicycle pump
- Scuba diving or deep water diving
What does Boyles law relate to?
Boyle’s law is a gas law that describes the relationship between the pressure and volume of gas for a mass and temperature. This law is the mechanism by which the human respiratory system functions.
What are real world situation with Boyles law?
The pressure in the pump has to always be higher than that which is inside the tire in order for more air to be pushed in. As the air temperature is more or less constant in that period of time, you can see a real live example of the Boyle’s law occurring in front of you. Soda Cans/Bottles: You may have noticed that whenever a person opens a ...
What is the formula of Boyles law?
We can use Boyle's law formula: p₂ = p₁ * V₁ / V₂ = 100 kPa * 2 m³ / 1 m³ = 200 kPa. After halving the volume, the internal pressure is doubled. This is a consequence of the fact that the product of the pressure and the volume must be constant during this process.

What is Boyle's law simple definition?
Definition of Boyle's law : a statement in physics: the volume of a gas at constant temperature varies inversely with the pressure exerted on it.
Is breathing an example of Boyle's law?
Boyle's law has application in human breathing. As the lungs expands, the volume inside the lungs increases and the pressure inside decreases (it follows Boyle's law). As the pressure is in lower concentration inside the body, the air moves inside the lungs from outsides.
How do you explain Boyle's law to a child?
0:265:25Chemistry: Boyle's Law (Gas Laws) with 2 examples | Homework TutorYouTubeStart of suggested clipEnd of suggested clipYou compress the gas. The particles of gas will run into the sides more often per second. So thatMoreYou compress the gas. The particles of gas will run into the sides more often per second. So that means higher pressure if you keep the amount of gas particles constant.
How does Boyle's law apply to lungs?
Boyle's law explains that pressure and volume are always inversely proportional at a given temperature of a gas. It explains that when the volume of the lung increases during inspiration, the pressure in the lung will decrease. This causes air at atmospheric pressure to rush in and fill the lung.
Is popcorn an example of Boyle's Law?
The Process of a Kernel Turning into a Popcorn Along with this, pressure decreases, which follows Boyle's law (pressure and volume are reverse.)
How do you prove Boyle's law?
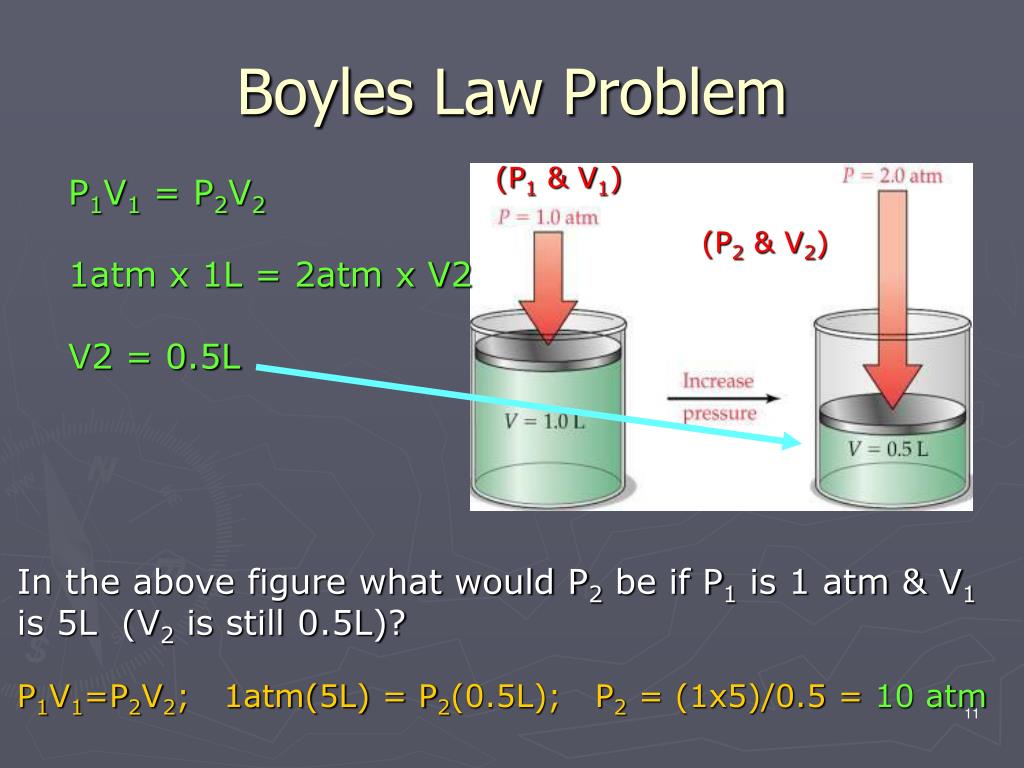
He made these observations by using Mercury in a J-tube and made measurements of the volume of the trapped gas at pressures both higher and lower then normal atmospheric pressure. Boyle expressed his results in a relationship that is known as Boyle's equation: P1V1 = P2V2 assuming the temperature remains constant.
Why is it called Boyle's law?
Boyle observed that the product of the pressure and volume are observed to be nearly constant. The product of pressure and volume is exactly a constant for an ideal gas. This relationship between pressure and volume is called Boyle's Law in his honor.
What is Boyle's law and write its equation?
Boyle's law states that "At constant temperature, the volume of a fixed mass of dry gas is inversely proportional to its pressure." Mathematical form. V∝1/P. V=K/P. PV=K.
How does Boyle’s law work?
Boyle’s law is a gas law that states that a gas’s pressure and volume are inversely proportional. When the temperature is kept constant, as volume...
Why is Boyle law important?
Boyle’s law is significant because it explains how gases behave. It proves beyond a shadow of a doubt that gas pressure and volume are inversely pr...
What is the formula for Boyle’s gas law?
The empirical relation asserts that the pressure (p) of a given quantity of gas changes inversely with its volume (v) at constant temperature; i.e....
What is a good example of Boyle’s Law?
A balloon is a good example of Boyle’s law in action. The balloon is inflated by blowing air into it; the pressure of the air pulls on the rubber,...
Can Boyle’s law be experimentally proven?
Boyle’s law is a connection between pressure and volume. It asserts that under constant temperature, the pressure of a specific quantity of gas is...
What is Boyle’s law?
Boyle’s law is a gas law given by the Anglo-Irish chemist Robert Boyle in 1662. He stated that the pressure exerted by a gas is inversely proportio...
What is the relationship between pressure and volume?
The pressure and volume are inversely proportional to each other under Boyle’s law. P ∝ (1/V)
Why does volume decrease when pressure is increased?
Volume decreases with increasing pressure because the gas particles come close to each other with increasing pressure. Similarly, volume increases...
What happens to pressure if the volume is doubled?
For a fixed mass of gas at a constant temperature, pressure is inversely proportional to volume. If the volume is doubled, the pressure will be hal...
What does Boyle's Law?
It provides the empirical relation between pressure and volume at constant temperature. That is p ∝ 1/V.
How do you calculate Boyle's Law?
Boyles law itself provides the relationship between volume and pressure and is used to carry out different experiments, you can use the equation p1...
How do you solve Boyle's law problems?
We use the equation p1V1 = p2V2 to solve the problems that require the application of Boyles law.
1. Breathing
During respiration, our lungs make use of Boyle’s law. While inhaling, the lungs are filled with air; therefore, they expand. The volume increases, hence the pressure level goes down. Similarly, when the lungs are evacuated of air, they shrink; therefore, the volume reduces and the pressure increases.
2. Inflating Tyres
Flat tyres lack proper shape and strength, which makes it difficult for a vehicle to move properly. When air is pressed into flat tyres with the help of an air pump, the air molecules get tightly packed. The more be air molecules present in the tyre, the more will be the pressure exerted on the walls of the tyre.
3. Soda bottle
A soda bottle, filled with a mixture of carbon-di-oxide and water, is one of the best examples to demonstrate Boyle’s law. When the soda can or bottle is sealed, it is difficult to compress. This is because the air molecules present inside the container are tightly packed and do not have space to move.
4. Working of a Syringe
A syringe is medical equipment that is used to insert or withdraw fluids. It consists of a cylinder to contain the fluid and a plunger to vary the pressure. When the plunger is pushed down, the volume of the fluid reduces, thereby increasing the pressure. Similarly, on pulling up the plunger, the volume is increased, and the pressure is reduced.
5. Spray Paint
Spray paints work on the basis of Boyle’s law. A significant amount of pressure is exerted by the paint molecules on the body of the can in which it is contained. When the top of the can is pressed, the volume inside the can gets reduced and the paint is thrown out with great pressure.
6. Spacesuits
Space does not consist of air or atmosphere. It has zero pressure as there is a vacuum in space. As per Boyle’s law, when a pressurized gas enters a vacuum region, it will expand infinitely. This is the reason why astronauts wear specially designed spacesuits.
7. Scuba Diving
One thing to keep in mind when a person goes underwater diving is that he must balance the volume and pressure relationship to avoid getting sick or hurt. When he/she enters or approaches the depth of the water body, he/she experiences high pressure. The high pressure increases the solubility of gases in the human blood.
What does Boyle's law mean?
As long as the temperature and number of moles of gas remain constant, Boyle's law means doubling the pressure of a gas halves its volume. Here are more examples of Boyle's law in action: When the plunger on a sealed syringe is pushed, the pressure increases and the volume decreases. Since the boiling point is dependent on pressure, ...
What is the law of gas?
Boyle's gas law states that the volume of a gas is inversely proportional to the pressure of the gas when the temperature is held constant.
Is volume proportional to pressure?
Volume's inversely proportional to pressure if temperature's constant. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. She has taught science courses at the high school, college, and graduate levels. Boyle's gas law states that the volume of a gas is inversely proportional to the pressure ...
Does Boyle's law change temperature?
Since the temperature doesn't change, Boyle's law can be used. Boyle's gas law can be expressed as:
What are some examples of Boyle's law?
Boyle’s law states that under isothermal conditions, the pressure of a fixed amount of a gas is inversely proportional to its volume. Here are some real-life examples are: 1 Pumping a tire tube with gas. 2 The air in our lungs 3 Syringe 4 A scuba diver
What is Boyle's law?
Boyle’s law states that under isothermal conditions , the pressure of a fixed amount of a gas is inversely proportional to its volume. Here are some real-life examples are: Pumping a tire tube with gas. The air in our lungs. Syringe.
What is the equation for Boyles law?
Ans. Boyles law itself provides the relationship between volume and pressure and is used to carry out different experiments, you can use the equation p1V1 = p2V2.
Why is Boyle's law of compressibility important?
This is because when a given mass of a gas is compressed, the same number of molecules occupy a smaller space. Hence we can obtain a relation between density and pressure by using this law.
Which law states that gas varies inversely with volume?
Boyle’s Law: This law comes under the topic/chapter States of Matter and provides the relation between gas and volume. The law states that “ at constant temperature, the pressure of a fixed amount (i.e., number of moles n) of gas varies inversely with its volume “.
What is the pressure of gas inversely proportional to volume?
According to Boyle’s Law, the pressure exerted by a fixed amount of gas (no. of moles) is inversely proportional to the volume, when the temperature is kept constant.
What Is Boyle's Law and Equation?
In 1662, Robert Boyle discovered the volume and pressure of gasses are inversely proportionate when held at a constant temperature. Put simply, when volume rises, pressure drops, and vice versa .
Why Is Boyle's Law Important?
Boyle's law is important because it tells us about the behavior of gasses. It explains, with certainty, that the pressure and volume of gas are inversely proportional to one another. So, if you push on gas, its volume becomes smaller and the pressure becomes higher.
How Did Boyle Come up With His Law?
Using a vacuum pump invented by Otto von Guericke in 1654, Boyle carried out experiments investigating the properties of air and the vacuum.
How Does Boyle's Law Relate to Breathing?
When it comes to the effects of Boyle's law on the body, the gas law specifically applies to the lungs.
What is the law of pressure?
Gay Lussac's law, or the pressure law, was discovered by Joseph Louis Gay-Lussac in 1809 and states that, for a given mass and constant volume of an ideal gas, the pressure exerted on the sides of its container is directly proportional to its absolute temperature. This means that pressure indicates temperature.
When was Charles' law of volume discovered?
Charle's law, or the law of volumes, was discovered in 1787 by Jaques Charles and states that for a give mass of an ideal gas at constant pressure, the volume is directly proportional to it's absolute temperature. This means that as the temperature of a gas increases, so does its volume.
Density & Pressure Relation with Boyles Law
Boyle’s experiments show that gases are very compressible when carried out in a quantitative manner. This is due to the fact that as a gas’s mass is reduced, the same number of molecules occupy a smaller space.
Conclusion
Despite the fact that Boyle’s law describes the behavior of an ideal gas, it may be applied to real gases at regular temperatures and pressures. Gases begin to depart from any version of the ideal gas law as temperature and pressure rise.
What are some examples of Boyle's law?
A few very interesting examples regarding Boyle’s Law in everyday life are discussed below: 1. Change of Pressure in a Syringe. A syringe is an everyday device used in a hospital to draw blood samples or give injections. When the plunger of the syringe is pulled back the volume of the syringe container increases, ...
What is Boyle's law?
Physical Science. Boyle’s law states that if the temperature of a gas is kept constant, the pressure if the gas is inversely proportional to the volume. Which suggests that in an ideal situation where the temperature does not change, if either the pressure or volume is increased the other one will decrease by the same proportion. Its formula is:
What are some examples of Boyle's law?
As the air temperature is more or less constant in that period of time, you can see a real live example of the Boyle’s law occurring in front of you. Soda Cans/Bottles: You may have noticed that whenever a person opens a can or bottle of soda, the cap or the lid is opened slowly, allowing the gas inside to escape at a controlled rate. ...
What is Boyle's law?
Respiration: Boyle’s law is essential for the human breathing process. As the muscles of the diaphragm contract, the decreased pressure causes the volume of the thoracic cavity to expand as you breathe in. When you breathe out, the volume of the thoracic cavity goes down, increasing the pressure on the lungs, and pushing air out.
Why is Boyle's law important?
Boyle's law is a very important gas law, which helps us closely understand the interrelation between the physical forces of pressure, volume, and temperature. In this article, we will look at a few examples of where this law comes into play in our daily lives.
What is the law of gas?
In the year 1662, physicist Robert Boyle propounded the Boyle’s law, which stated that the pressure and volume of a gas were inversely proportionate when its temperature was kept constant. In other words, at a constant temperature, when the volume of a gas goes up, the pressure goes down, while the volume drops when the pressure rises. The equation of this phenomenon, V1/V2=P2/P1 (at constant temperature), where V1 is the initial volume, V2 is the modified volume, P1 is the starting pressure, and P2 is the modified pressure, is nowadays explained as Boyle’s Law , and is an important part of the ideal gas law. This law can be observed everywhere in our daily lives, in the mechanisms of several everyday objects. As such, let us look at a few examples of the Boyle’s law in real life.
Is Boyle's law used in the real world?
As you can see, Boyle’s law has immense practical uses in the real world, apart from the theoretical studies of ideal and real gases, and still forms a crucial part of research that has anything to do with gases. « Previous Post. Next Post ».
Worked Example Problem
The sections on the General Properties of Gases and Ideal Gas Law Problems may also be helpful when attempting to work Boyle's Law problems .
Source
Levine, Ira N. (1978). Physical Chemistry. University of Brooklyn: McGraw-Hill.

What Is Boyle's Law and equation?
How Did Boyle Come Up with His Law?
- Using a vacuum pump invented by Otto von Guericke in 1654, Boyle carried out experiments investigating the properties of air and the vacuum. During his experiments, he stumbled upon the greatest achievement of his life. By using a J-shaped glass tube that had air at the tip of the curve, Boyle altered the weight of the air using mercury and, as he did so, he saw that the space of air a…
Why Is Boyle's Law Important?
- Boyle's law is important because it tells us about the behavior of gasses. It explains, with certainty, that the pressure and volume of gas are inversely proportional to one another. So, if you push on gas, its volume becomes smaller and the pressure becomes higher.
Examples of Boyle's Law in Life
- You have probably been well-acquainted with Boyle's law for most of your life without realizing it. We experience examples of this law on a regular basis. The first example is a rather common one, assuming you have filled a tire with air before. Generally, you fill a tire with somewhere between 30 to 35 PSI (pounds per square inch) of compressed ai...
Spray Paint
- While there are a couple different types of aerosol cans, some being a little more elaborate than other, they all rely on the same basic principle: Boyle's law. Before you spray a can of paint, you are supposed to shake it up for a while as a ball bearing rattles around inside. There are two substances inside the can: one is your product (paint for example), and the other is a gas that ca…
The Syringe
- This mechanism is far more simple than a can of spray paint. Syringes of all types utilize Boyle's law on a very basic level. When you pull the plunger out on a syringe, it causes the volume within the chamber to increase. As we know, this causes the pressure to do the opposite, which then creates a vacuum. When a syringe is empty, the vacuum within the chamber sucks fluid in throu…
The Soda Can Or Bottle
- Typically when we open a bottle of soda, we slowly turn the cap to allow the air to escape before we completely remove the lid. We do this because we've learned over time that twisting it open too fast causes it to fizz up and spill all over. This happens because the liquid is pumped full of carbon dioxide, causing it to bubble up as the CO2 makes its escape. When a soda bottle is fille…
The Bends
- Any properly trained scuba diver knows when they are ascending from deep waters, a slow ascension is critical. Our bodies are built for and accustomed to living in the normal pressure of our lower atmosphere. As a diver goes deeper underwater, that pressure begins to increase. Water is heavy, after all. With the increasing pressure causing a decrease in volume, nitrogen ga…
What Is The Ideal Gas Law?
- Since it is hard to exactly describe a real gas, scientists created the concept of an ideal gas. The ideal gas law refers to a hypothetical gas that follows the rules listed below: 1. Ideal gas molecules do not attract or repel each other. The only interaction between ideal gas molecules would be an elastic collision with each other or with the walls of the container. 2. Ideal gas mole…
How Does Boyle's Law Relate to Breathing?
- When it comes to the effects of Boyle's law on the body, the gas law specifically applies to the lungs. When a person breathes in, their lung volume increases and the pressure within decreases. Since air always moves from areas of high pressure to areas of low pressure, air is drawn into the lungs. The opposite happens when a person exhales. Since the lung volume decreases, the pres…